| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1629739 | Journal of Iron and Steel Research, International | 2011 | 4 Pages |
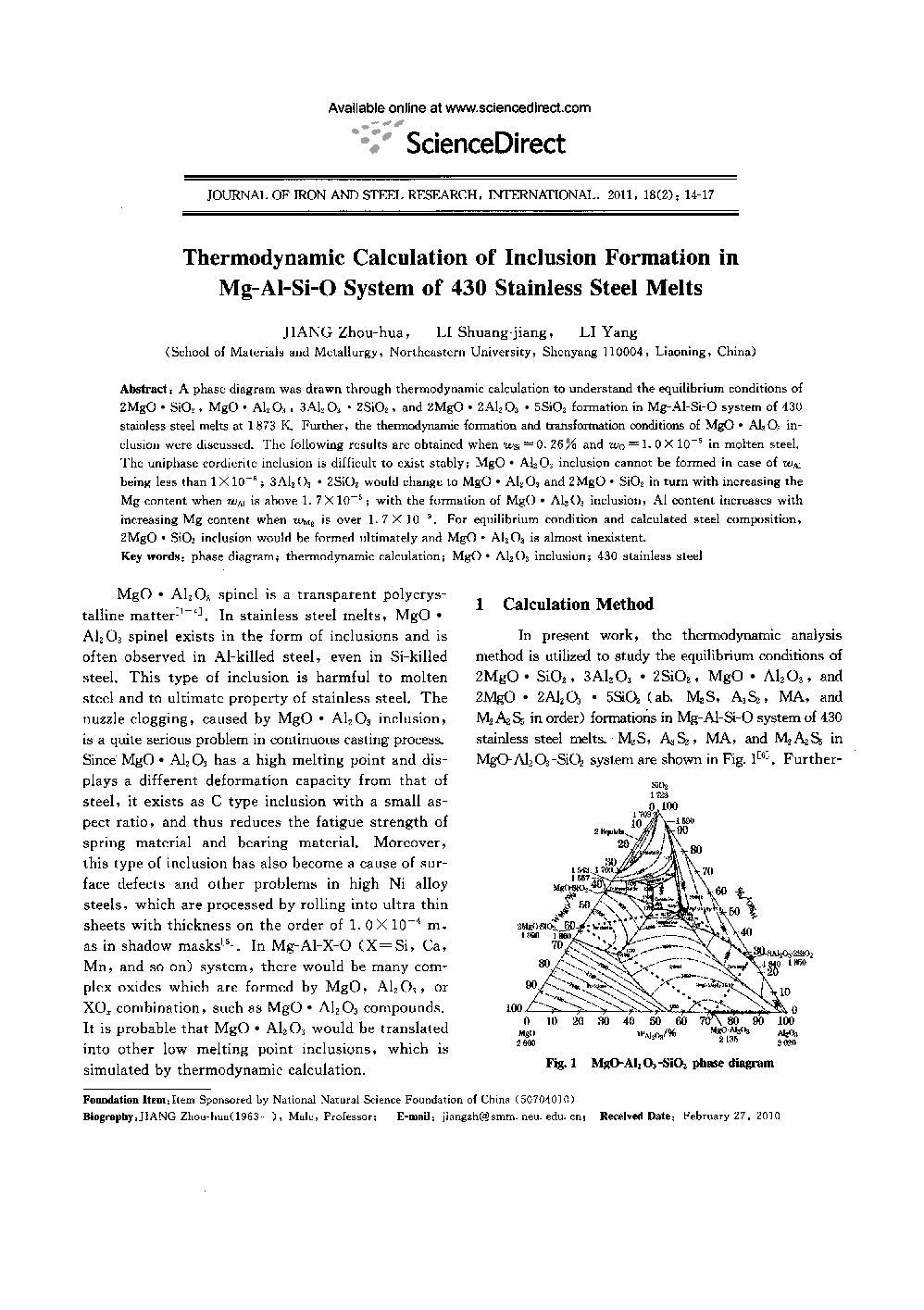
A phase diagram was drawn through thermodynamic calculation to understand the equilibrium conditions of 2MgO · SiO2, MgO · Al2O3, 3Al2O3 · 2SiO2, and 2MgO · 2Al2O3 5SiO2 formation in Mg-Al-Si-O system of 430 stainless steel melts at 1873 K. Further, the thermodynamic formation and transformation conditions of MgO · A1203 inclusion were discussed. The following results are obtained when wsi, = 0.26% and wo = 1.0 × 10−5 in molten steel. The uniphase cordierite inclusion is difficult to exist stably; MgO · Al2O3 inclusion cannot be formed in case of wAl being less than 1 × 10−6; 3Al2O3 2SiO2 would change to MgO Al2O3 and 2MgO SiO2 in turn with increasing the Mg content when wAl is above 1.7 × 10−6; with the formation of MgO · Al2O3 inclusion, Al content increases with increasing Mg content when wMg is over 1.7 × 10−9. For equilibrium condition and calculated steel composition, MgO · SiO2 inclusion would be formed ultimately and MgO · Al2O3 is almost inexistent.