| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 167449 | Chinese Journal of Chemical Engineering | 2011 | 7 Pages |
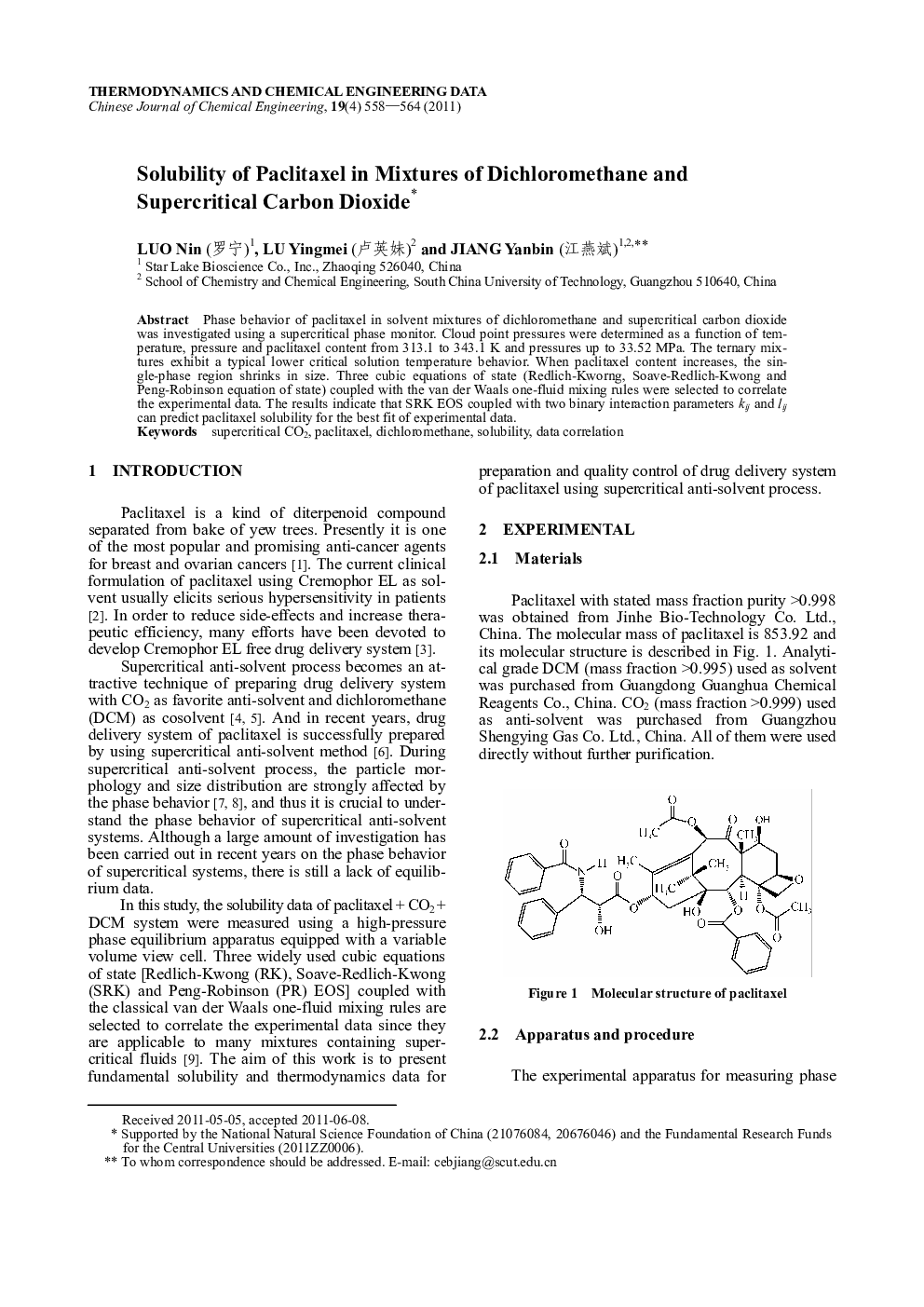
Phase behavior of paclitaxel in solvent mixtures of dichloromethane and supercritical carbon dioxide was investigated using a supercritical phase monitor. Cloud point pressures were determined as a function of temperature, pressure and paclitaxel content from 313.1 to 343.1 K and pressures up to 33.52 MPa. The ternary mixtures exhibit a typical lower critical solution temperature behavior. When paclitaxel content increases, the single-phase region shrinks in size. Three cubic equations of state (Redlich-Kworng, Soave-Redlich-Kwong and Peng-Robinson equation of state) coupled with the van der Waals one-fluid mixing rules were selected to correlate the experimental data. The results indicate that SRK EOS coupled with two binary interaction parameters kij and lij can predict paclitaxel solubility for the best fit of experimental data.