| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2035034 | Cell | 2016 | 14 Pages |
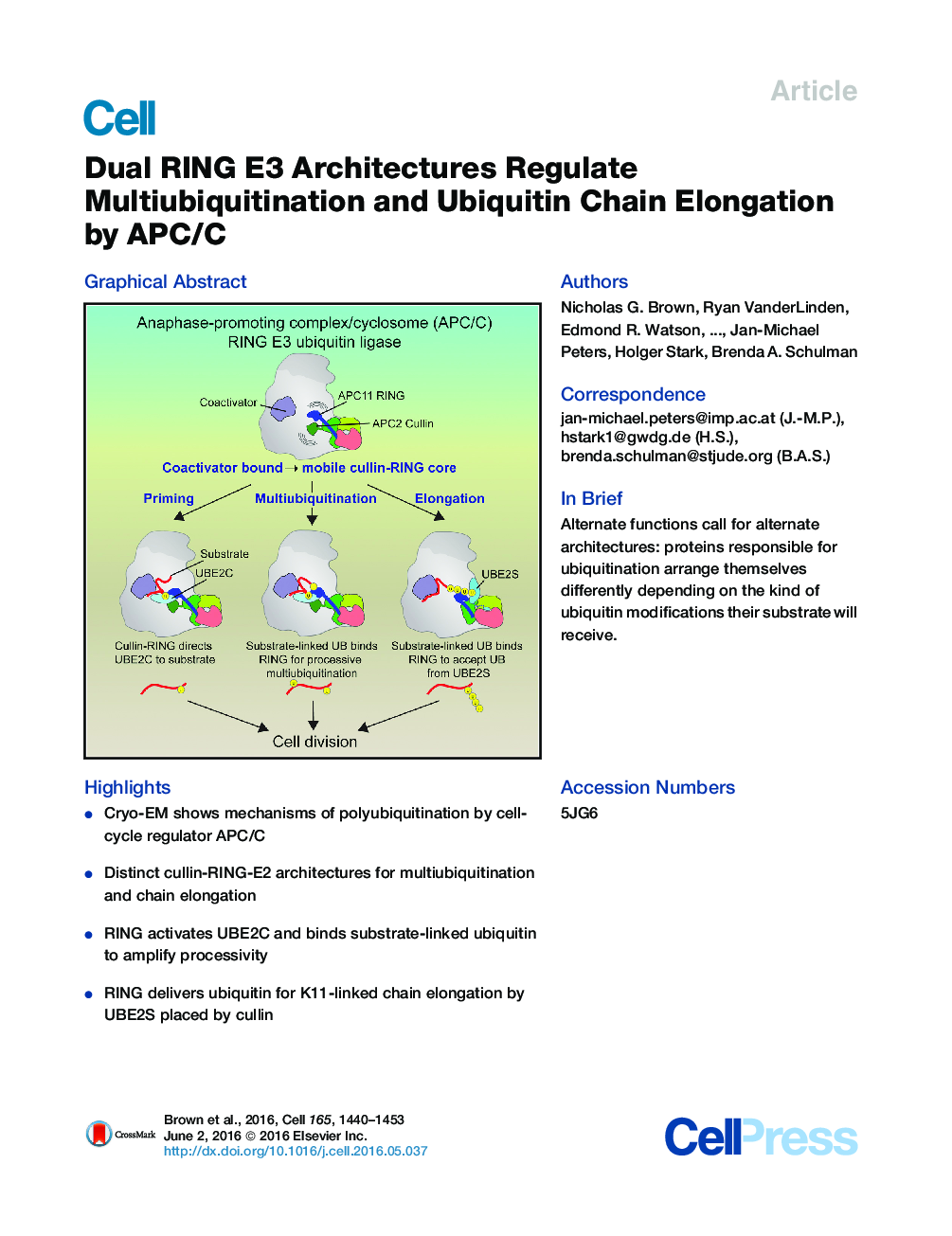
•Cryo-EM shows mechanisms of polyubiquitination by cell-cycle regulator APC/C•Distinct cullin-RING-E2 architectures for multiubiquitination and chain elongation•RING activates UBE2C and binds substrate-linked ubiquitin to amplify processivity•RING delivers ubiquitin for K11-linked chain elongation by UBE2S placed by cullin
SummaryProtein ubiquitination involves E1, E2, and E3 trienzyme cascades. E2 and RING E3 enzymes often collaborate to first prime a substrate with a single ubiquitin (UB) and then achieve different forms of polyubiquitination: multiubiquitination of several sites and elongation of linkage-specific UB chains. Here, cryo-EM and biochemistry show that the human E3 anaphase-promoting complex/cyclosome (APC/C) and its two partner E2s, UBE2C (aka UBCH10) and UBE2S, adopt specialized catalytic architectures for these two distinct forms of polyubiquitination. The APC/C RING constrains UBE2C proximal to a substrate and simultaneously binds a substrate-linked UB to drive processive multiubiquitination. Alternatively, during UB chain elongation, the RING does not bind UBE2S but rather lures an evolving substrate-linked UB to UBE2S positioned through a cullin interaction to generate a Lys11-linked chain. Our findings define mechanisms of APC/C regulation, and establish principles by which specialized E3–E2–substrate-UB architectures control different forms of polyubiquitination.
Graphical AbstractFigure optionsDownload full-size imageDownload high-quality image (234 K)Download as PowerPoint slide