| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2035036 | Cell | 2016 | 12 Pages |
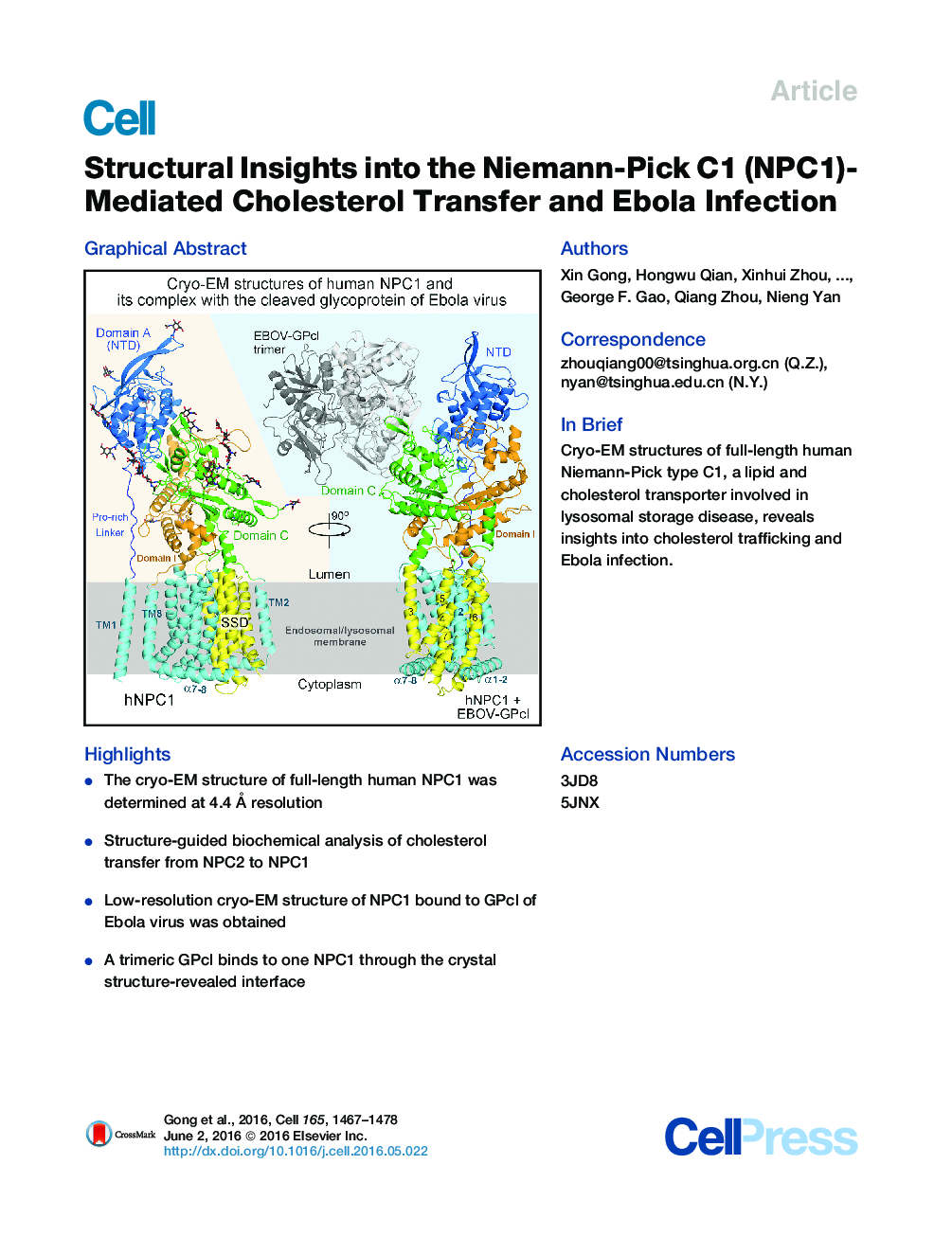
•The cryo-EM structure of full-length human NPC1 was determined at 4.4 Å resolution•Structure-guided biochemical analysis of cholesterol transfer from NPC2 to NPC1•Low-resolution cryo-EM structure of NPC1 bound to GPcl of Ebola virus was obtained•A trimeric GPcl binds to one NPC1 through the crystal structure-revealed interface
SummaryNiemann-Pick disease type C (NPC) is associated with mutations in NPC1 and NPC2, whose gene products are key players in the endosomal/lysosomal egress of low-density lipoprotein-derived cholesterol. NPC1 is also the intracellular receptor for Ebola virus (EBOV). Here, we present a 4.4 Å structure of full-length human NPC1 and a low-resolution reconstruction of NPC1 in complex with the cleaved glycoprotein (GPcl) of EBOV, both determined by single-particle electron cryomicroscopy. NPC1 contains 13 transmembrane segments (TMs) and three distinct lumenal domains A (also designated NTD), C, and I. TMs 2–13 exhibit a typical resistance-nodulation-cell division fold, among which TMs 3–7 constitute the sterol-sensing domain conserved in several proteins involved in cholesterol metabolism and signaling. A trimeric EBOV-GPcl binds to one NPC1 monomer through the domain C. Our structural and biochemical characterizations provide an important framework for mechanistic understanding of NPC1-mediated intracellular cholesterol trafficking and Ebola virus infection.
Graphical AbstractFigure optionsDownload full-size imageDownload high-quality image (450 K)Download as PowerPoint slide