| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2035348 | Cell | 2015 | 10 Pages |
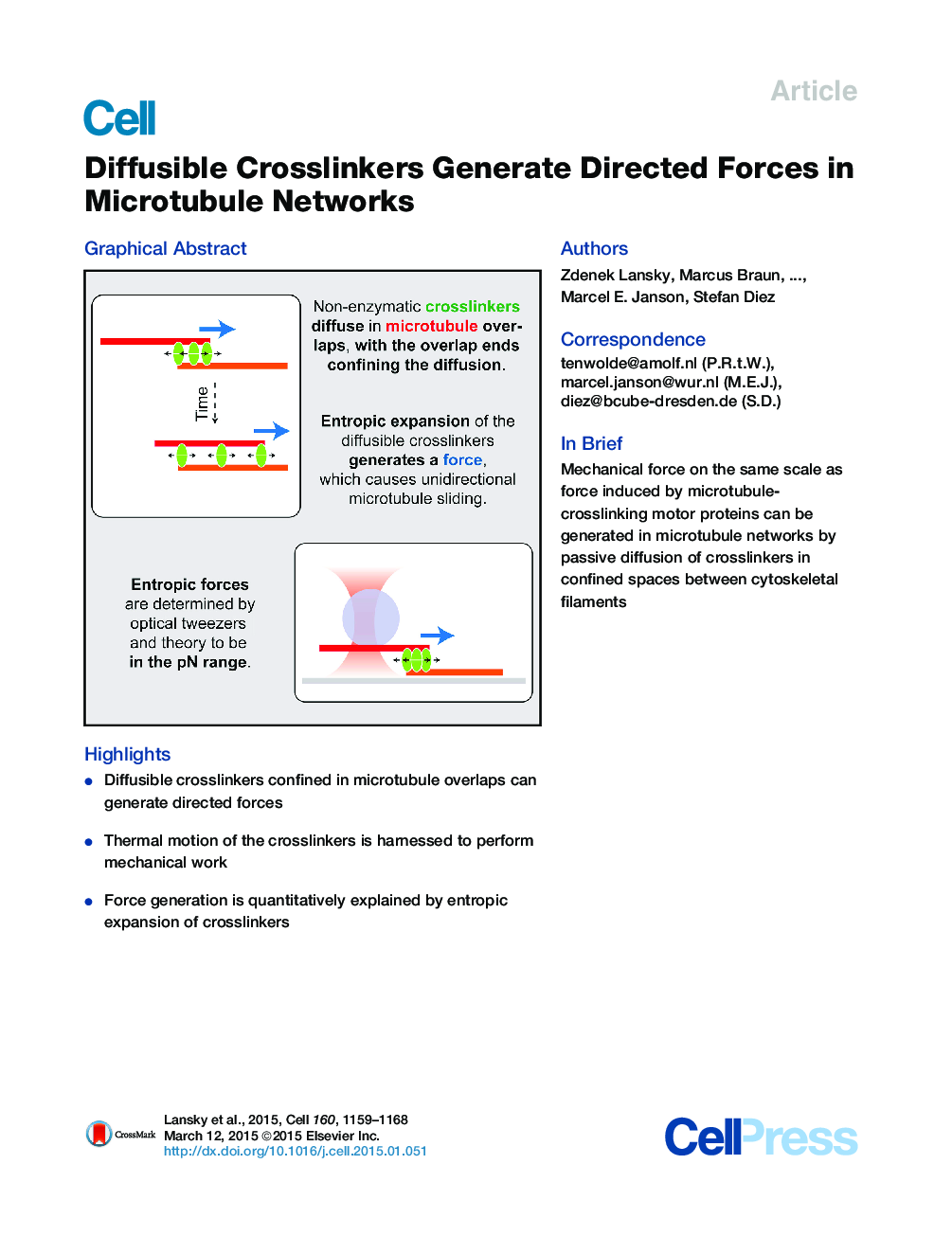
•Diffusible crosslinkers confined in microtubule overlaps can generate directed forces•Thermal motion of the crosslinkers is harnessed to perform mechanical work•Force generation is quantitatively explained by entropic expansion of crosslinkers
SummaryCytoskeletal remodeling is essential to eukaryotic cell division and morphogenesis. The mechanical forces driving the restructuring are attributed to the action of molecular motors and the dynamics of cytoskeletal filaments, which both consume chemical energy. By contrast, non-enzymatic filament crosslinkers are regarded as mere friction-generating entities. Here, we experimentally demonstrate that diffusible microtubule crosslinkers of the Ase1/PRC1/Map65 family generate directed microtubule sliding when confined between partially overlapping microtubules. The Ase1-generated forces, directly measured by optical tweezers to be in the piconewton-range, were sufficient to antagonize motor-protein driven microtubule sliding. Force generation is quantitatively explained by the entropic expansion of confined Ase1 molecules diffusing within the microtubule overlaps. The thermal motion of crosslinkers is thus harnessed to generate mechanical work analogous to compressed gas propelling a piston in a cylinder. As confinement of diffusible proteins is ubiquitous in cells, the associated entropic forces are likely of importance for cellular mechanics beyond cytoskeletal networks.
Graphical AbstractFigure optionsDownload full-size imageDownload high-quality image (167 K)Download as PowerPoint slide