| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 205007 | Fuel | 2016 | 7 Pages |
•Novel supported rare earth double perovskite oxide was synthesized by three methods.•La2CoMnO6/CeO2 (precipitation) shows the best activity.•Co3+, Mn4+ and lattice oxygen play a crucial role in methane catalytic combustion.
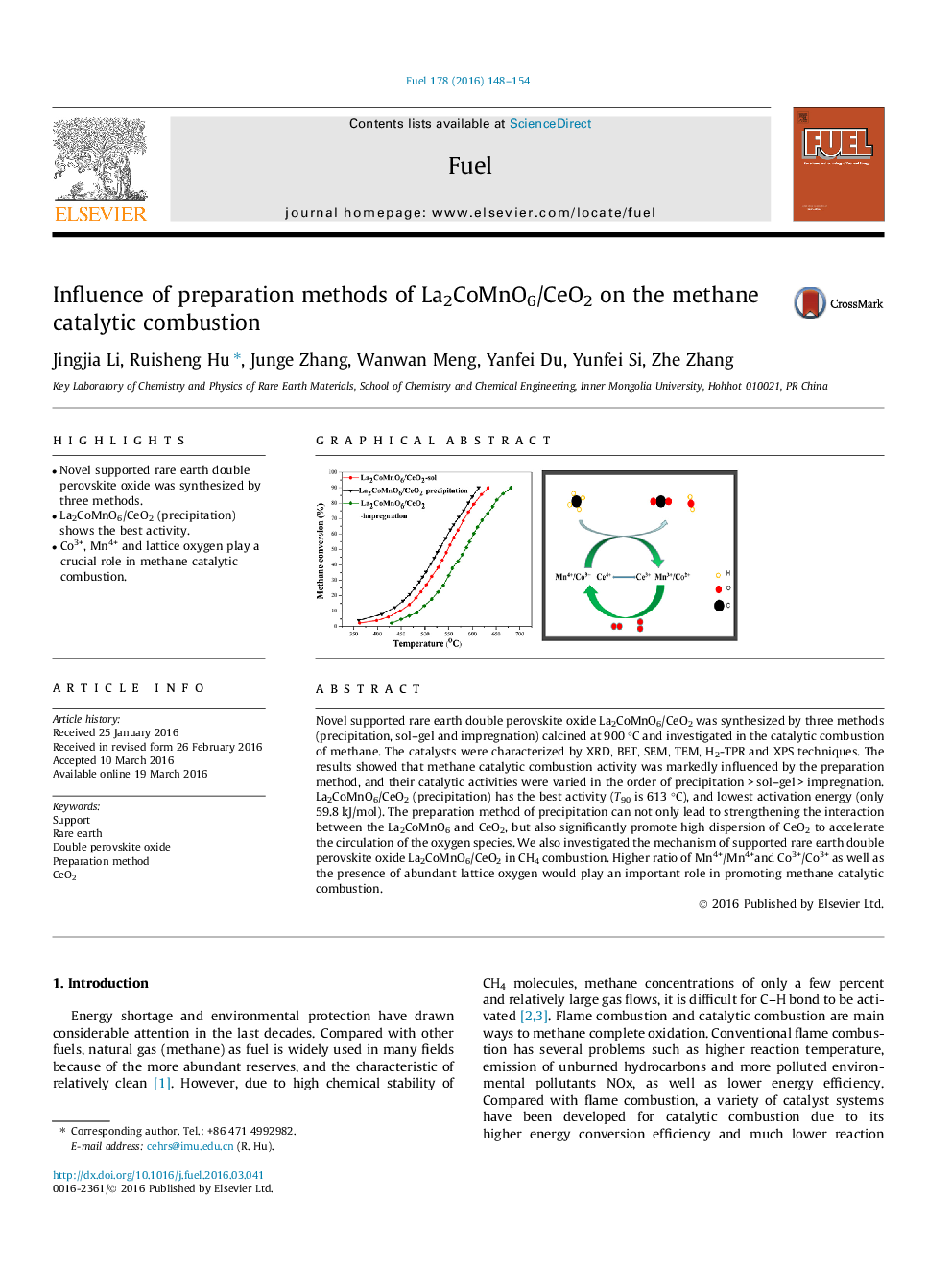
Novel supported rare earth double perovskite oxide La2CoMnO6/CeO2 was synthesized by three methods (precipitation, sol–gel and impregnation) calcined at 900 °C and investigated in the catalytic combustion of methane. The catalysts were characterized by XRD, BET, SEM, TEM, H2-TPR and XPS techniques. The results showed that methane catalytic combustion activity was markedly influenced by the preparation method, and their catalytic activities were varied in the order of precipitation > sol–gel > impregnation. La2CoMnO6/CeO2 (precipitation) has the best activity (T90 is 613 °C), and lowest activation energy (only 59.8 kJ/mol). The preparation method of precipitation can not only lead to strengthening the interaction between the La2CoMnO6 and CeO2, but also significantly promote high dispersion of CeO2 to accelerate the circulation of the oxygen species. We also investigated the mechanism of supported rare earth double perovskite oxide La2CoMnO6/CeO2 in CH4 combustion. Higher ratio of Mn4+/Mn4+and Co3+/Co3+ as well as the presence of abundant lattice oxygen would play an important role in promoting methane catalytic combustion.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide