| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 205114 | Fuel | 2016 | 11 Pages |
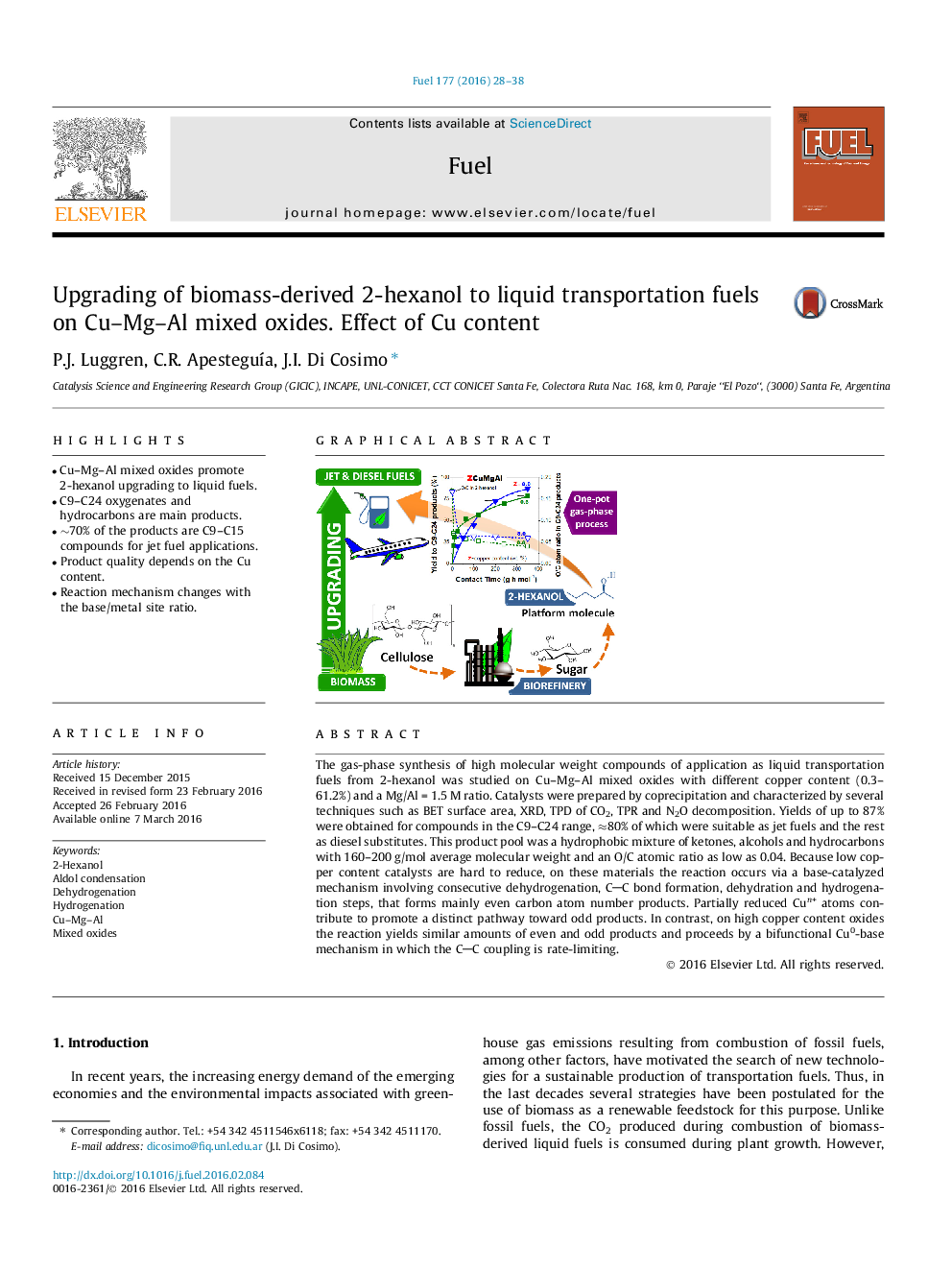
•Cu–Mg–Al mixed oxides promote 2-hexanol upgrading to liquid fuels.•C9–C24 oxygenates and hydrocarbons are main products.•∼70% of the products are C9–C15 compounds for jet fuel applications.•Product quality depends on the Cu content.•Reaction mechanism changes with the base/metal site ratio.
The gas-phase synthesis of high molecular weight compounds of application as liquid transportation fuels from 2-hexanol was studied on Cu–Mg–Al mixed oxides with different copper content (0.3–61.2%) and a Mg/Al = 1.5 M ratio. Catalysts were prepared by coprecipitation and characterized by several techniques such as BET surface area, XRD, TPD of CO2, TPR and N2O decomposition. Yields of up to 87% were obtained for compounds in the C9–C24 range, ≈80% of which were suitable as jet fuels and the rest as diesel substitutes. This product pool was a hydrophobic mixture of ketones, alcohols and hydrocarbons with 160–200 g/mol average molecular weight and an O/C atomic ratio as low as 0.04. Because low copper content catalysts are hard to reduce, on these materials the reaction occurs via a base-catalyzed mechanism involving consecutive dehydrogenation, CC bond formation, dehydration and hydrogenation steps, that forms mainly even carbon atom number products. Partially reduced Cun+ atoms contribute to promote a distinct pathway toward odd products. In contrast, on high copper content oxides the reaction yields similar amounts of even and odd products and proceeds by a bifunctional Cu0-base mechanism in which the CC coupling is rate-limiting.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide