| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 205389 | Fuel | 2016 | 8 Pages |
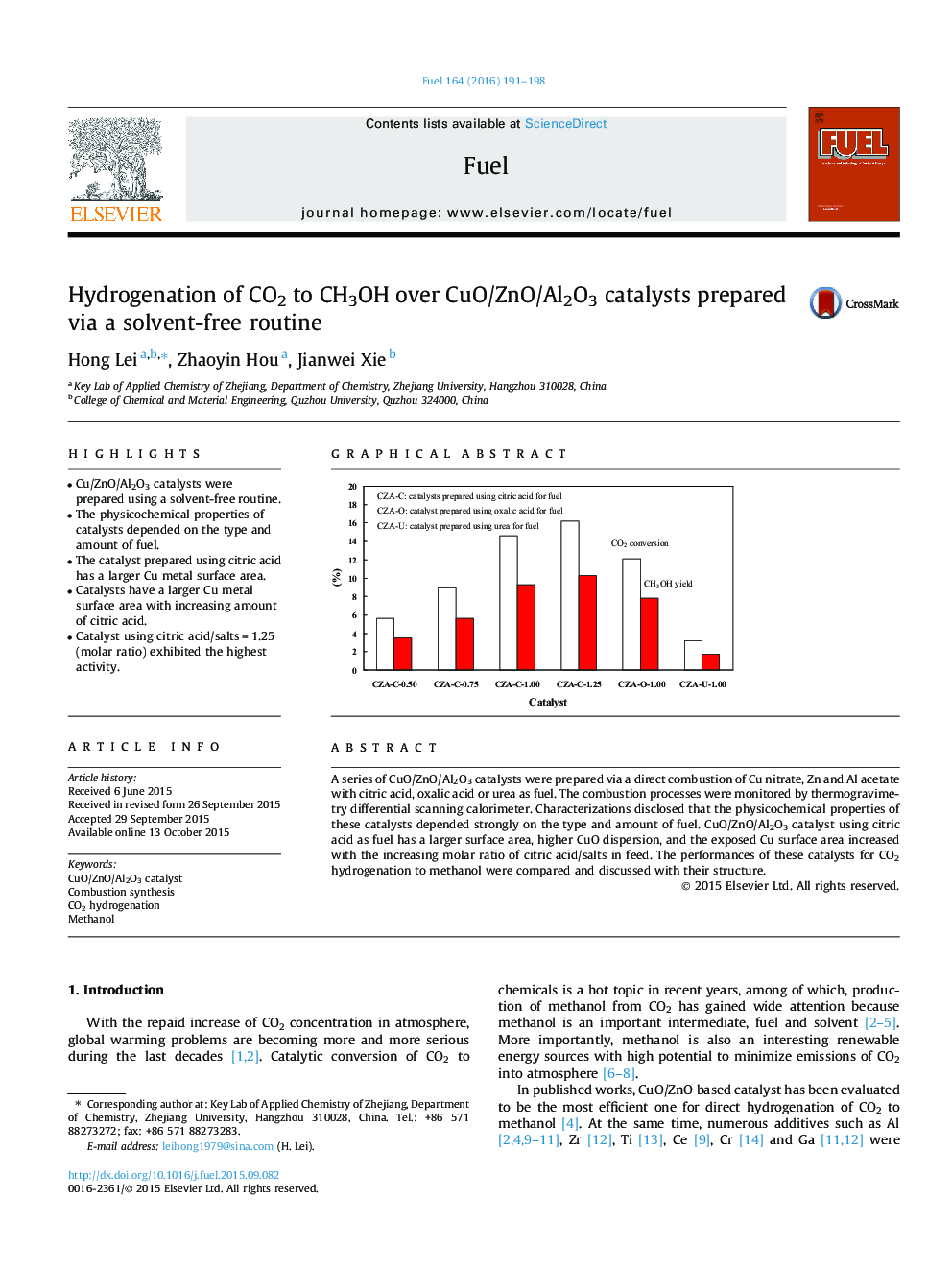
•Cu/ZnO/Al2O3 catalysts were prepared using a solvent-free routine.•The physicochemical properties of catalysts depended on the type and amount of fuel.•The catalyst prepared using citric acid has a larger Cu metal surface area.•Catalysts have a larger Cu metal surface area with increasing amount of citric acid.•Catalyst using citric acid/salts = 1.25 (molar ratio) exhibited the highest activity.
A series of CuO/ZnO/Al2O3 catalysts were prepared via a direct combustion of Cu nitrate, Zn and Al acetate with citric acid, oxalic acid or urea as fuel. The combustion processes were monitored by thermogravimetry differential scanning calorimeter. Characterizations disclosed that the physicochemical properties of these catalysts depended strongly on the type and amount of fuel. CuO/ZnO/Al2O3 catalyst using citric acid as fuel has a larger surface area, higher CuO dispersion, and the exposed Cu surface area increased with the increasing molar ratio of citric acid/salts in feed. The performances of these catalysts for CO2 hydrogenation to methanol were compared and discussed with their structure.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide