| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 205487 | Fuel | 2016 | 9 Pages |
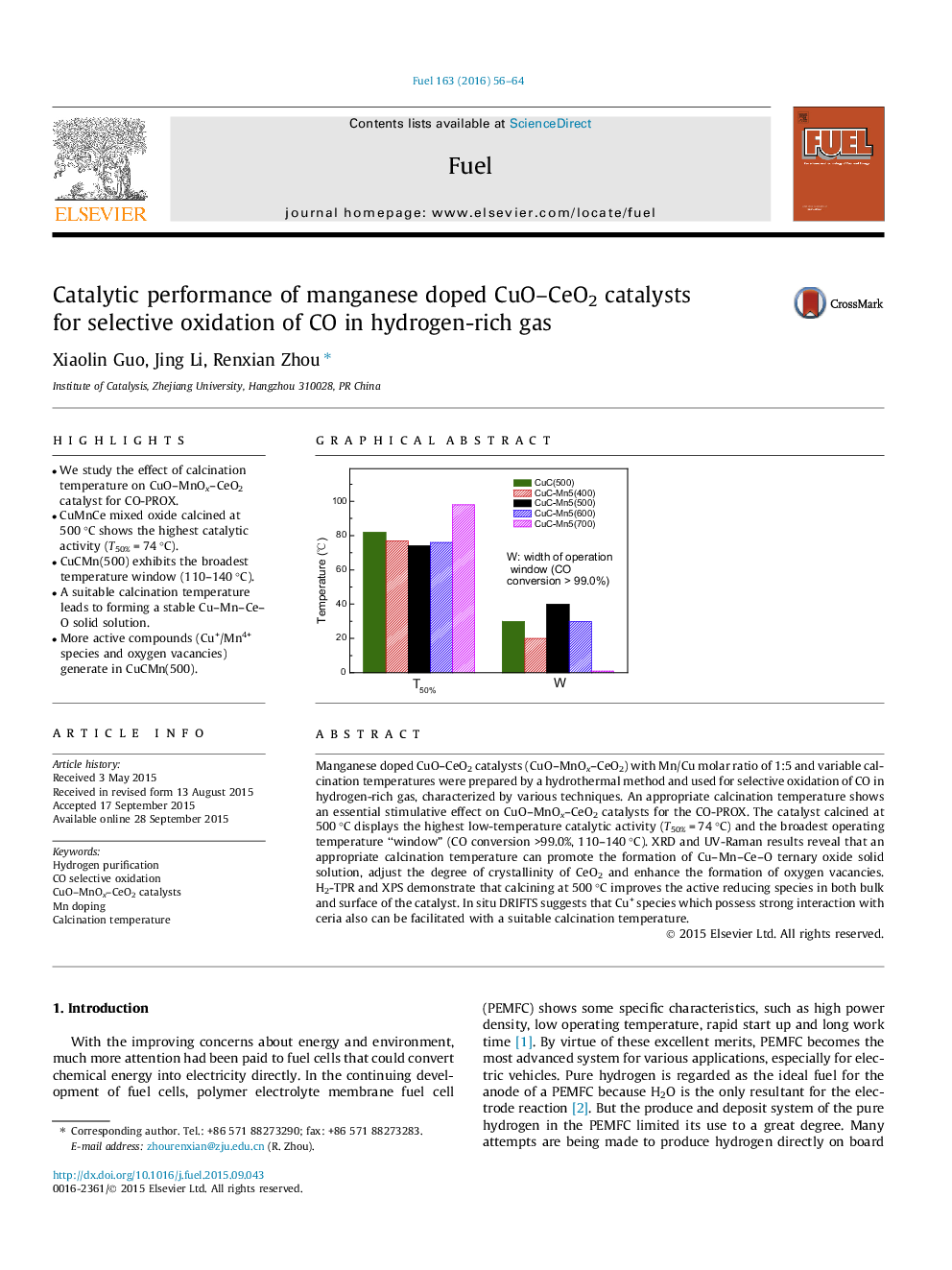
•We study the effect of calcination temperature on CuO–MnOx–CeO2 catalyst for CO-PROX.•CuMnCe mixed oxide calcined at 500 °C shows the highest catalytic activity (T50% = 74 °C).•CuCMn(500) exhibits the broadest temperature window (110–140 °C).•A suitable calcination temperature leads to forming a stable Cu–Mn–Ce–O solid solution.•More active compounds (Cu+/Mn4+ species and oxygen vacancies) generate in CuCMn(500).
Manganese doped CuO–CeO2 catalysts (CuO–MnOx–CeO2) with Mn/Cu molar ratio of 1:5 and variable calcination temperatures were prepared by a hydrothermal method and used for selective oxidation of CO in hydrogen-rich gas, characterized by various techniques. An appropriate calcination temperature shows an essential stimulative effect on CuO–MnOx–CeO2 catalysts for the CO-PROX. The catalyst calcined at 500 °C displays the highest low-temperature catalytic activity (T50% = 74 °C) and the broadest operating temperature “window” (CO conversion >99.0%, 110–140 °C). XRD and UV-Raman results reveal that an appropriate calcination temperature can promote the formation of Cu–Mn–Ce–O ternary oxide solid solution, adjust the degree of crystallinity of CeO2 and enhance the formation of oxygen vacancies. H2-TPR and XPS demonstrate that calcining at 500 °C improves the active reducing species in both bulk and surface of the catalyst. In situ DRIFTS suggests that Cu+ species which possess strong interaction with ceria also can be facilitated with a suitable calcination temperature.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide