| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 205530 | Fuel | 2015 | 13 Pages |
•A numerical model for the combustion of single lithium particles is developed based on experimental findings.•The model considers lithium’s gas-phase reaction and surface combustion with CO2.•The gas-phase reaction terminates due to a product layer on the particle surface.•Simulation and experiments are in good agreement concerning particle temperatures and conversion rates.
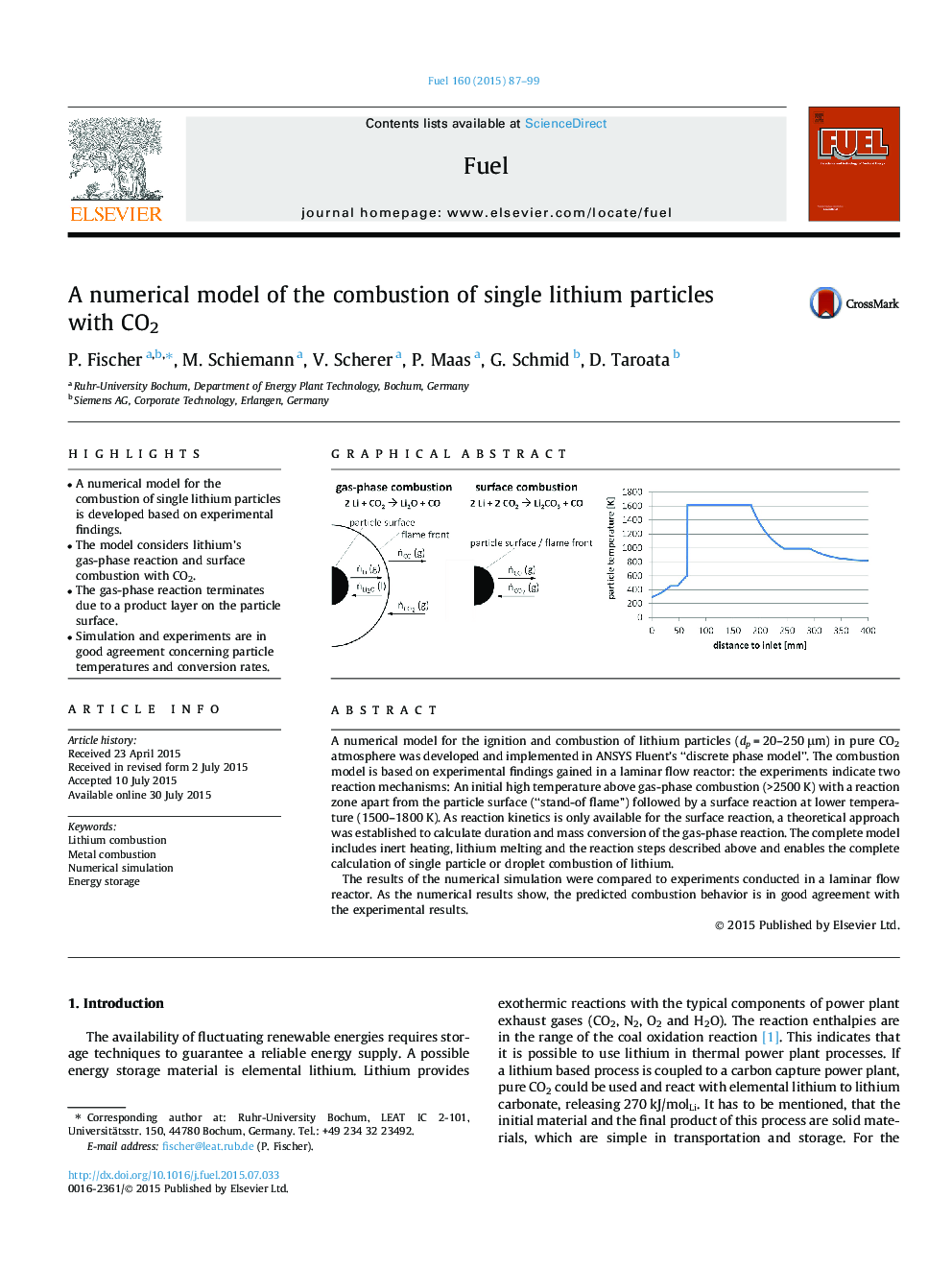
A numerical model for the ignition and combustion of lithium particles (dp = 20–250 μm) in pure CO2 atmosphere was developed and implemented in ANSYS Fluent’s “discrete phase model”. The combustion model is based on experimental findings gained in a laminar flow reactor: the experiments indicate two reaction mechanisms: An initial high temperature above gas-phase combustion (>2500 K) with a reaction zone apart from the particle surface (“stand-of flame”) followed by a surface reaction at lower temperature (1500–1800 K). As reaction kinetics is only available for the surface reaction, a theoretical approach was established to calculate duration and mass conversion of the gas-phase reaction. The complete model includes inert heating, lithium melting and the reaction steps described above and enables the complete calculation of single particle or droplet combustion of lithium.The results of the numerical simulation were compared to experiments conducted in a laminar flow reactor. As the numerical results show, the predicted combustion behavior is in good agreement with the experimental results.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide