| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 205638 | Fuel | 2015 | 9 Pages |
•Sodium halides are methane hydrate promoters and inhibitors.•Promoter capability decreases with decreasing the ion size.•Our findings are explained by hydrophobic hydration.•Gas hydrate promotion by halides is owing to their hydrophobic nature.•Salt recovery into gas hydrates is significant.
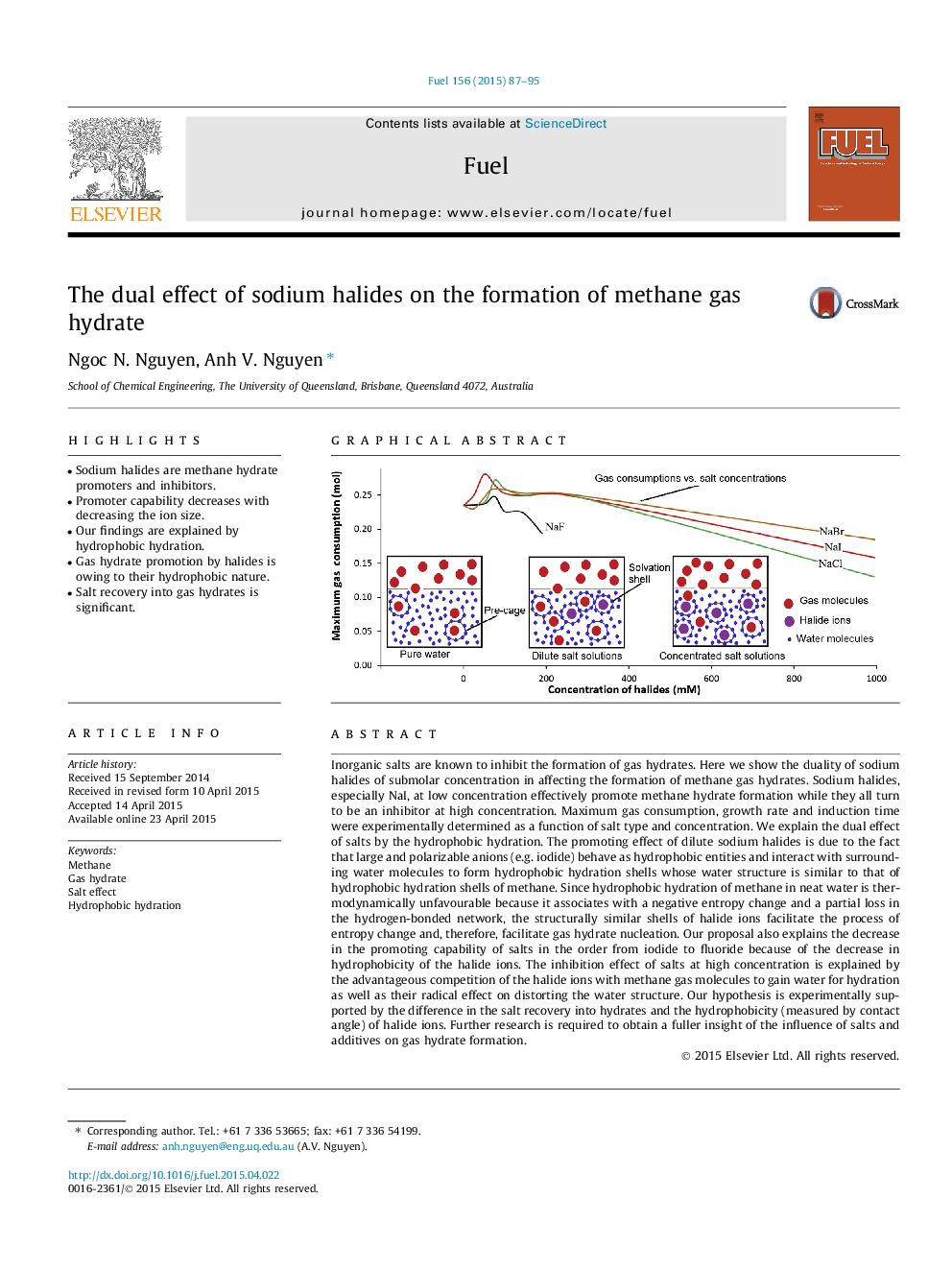
Inorganic salts are known to inhibit the formation of gas hydrates. Here we show the duality of sodium halides of submolar concentration in affecting the formation of methane gas hydrates. Sodium halides, especially NaI, at low concentration effectively promote methane hydrate formation while they all turn to be an inhibitor at high concentration. Maximum gas consumption, growth rate and induction time were experimentally determined as a function of salt type and concentration. We explain the dual effect of salts by the hydrophobic hydration. The promoting effect of dilute sodium halides is due to the fact that large and polarizable anions (e.g. iodide) behave as hydrophobic entities and interact with surrounding water molecules to form hydrophobic hydration shells whose water structure is similar to that of hydrophobic hydration shells of methane. Since hydrophobic hydration of methane in neat water is thermodynamically unfavourable because it associates with a negative entropy change and a partial loss in the hydrogen-bonded network, the structurally similar shells of halide ions facilitate the process of entropy change and, therefore, facilitate gas hydrate nucleation. Our proposal also explains the decrease in the promoting capability of salts in the order from iodide to fluoride because of the decrease in hydrophobicity of the halide ions. The inhibition effect of salts at high concentration is explained by the advantageous competition of the halide ions with methane gas molecules to gain water for hydration as well as their radical effect on distorting the water structure. Our hypothesis is experimentally supported by the difference in the salt recovery into hydrates and the hydrophobicity (measured by contact angle) of halide ions. Further research is required to obtain a fuller insight of the influence of salts and additives on gas hydrate formation.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide