| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 205703 | Fuel | 2015 | 9 Pages |
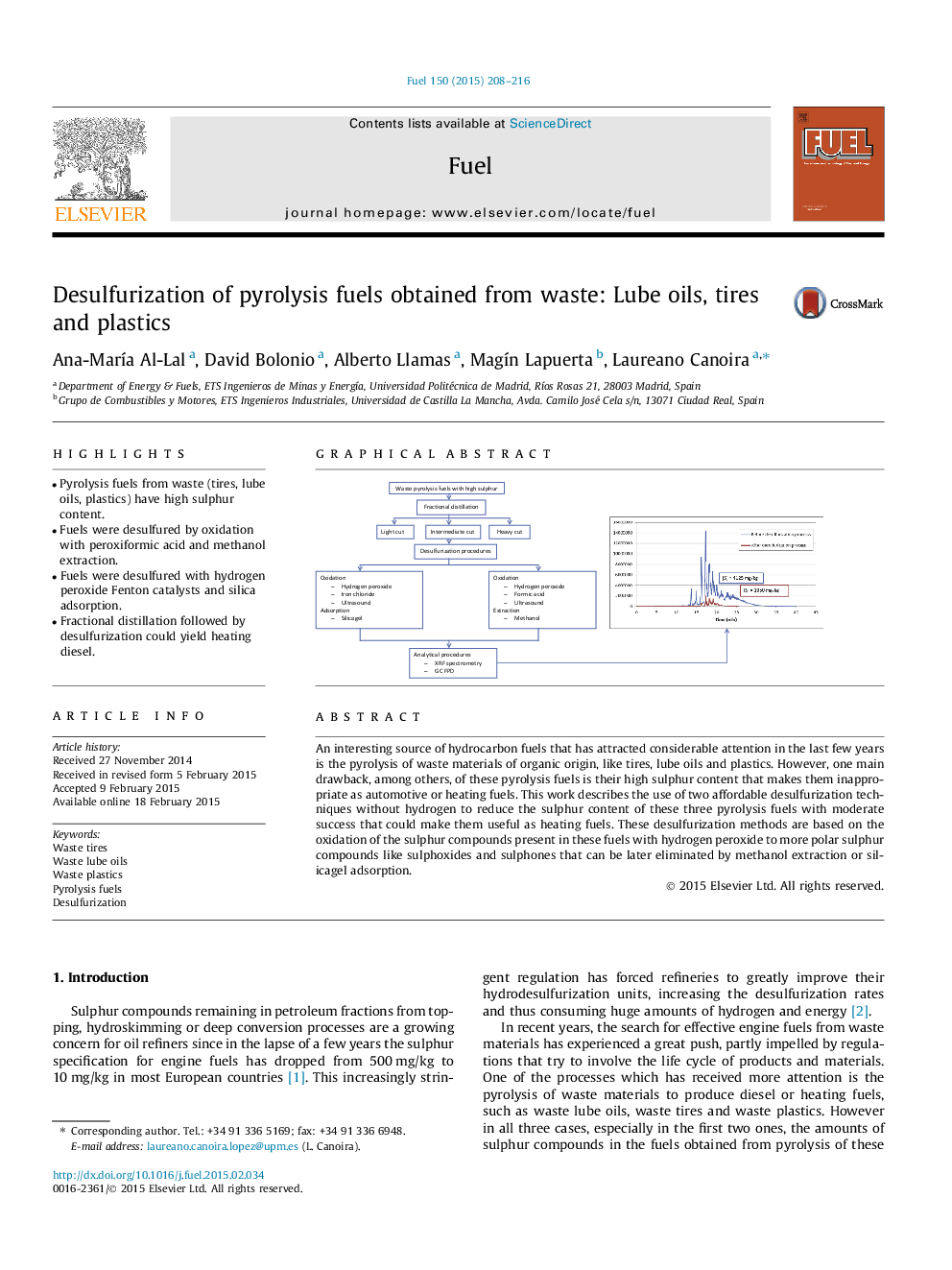
•Pyrolysis fuels from waste (tires, lube oils, plastics) have high sulphur content.•Fuels were desulfured by oxidation with peroxiformic acid and methanol extraction.•Fuels were desulfured with hydrogen peroxide Fenton catalysts and silica adsorption.•Fractional distillation followed by desulfurization could yield heating diesel.
An interesting source of hydrocarbon fuels that has attracted considerable attention in the last few years is the pyrolysis of waste materials of organic origin, like tires, lube oils and plastics. However, one main drawback, among others, of these pyrolysis fuels is their high sulphur content that makes them inappropriate as automotive or heating fuels. This work describes the use of two affordable desulfurization techniques without hydrogen to reduce the sulphur content of these three pyrolysis fuels with moderate success that could make them useful as heating fuels. These desulfurization methods are based on the oxidation of the sulphur compounds present in these fuels with hydrogen peroxide to more polar sulphur compounds like sulphoxides and sulphones that can be later eliminated by methanol extraction or silicagel adsorption.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide