| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 205993 | Fuel | 2014 | 10 Pages |
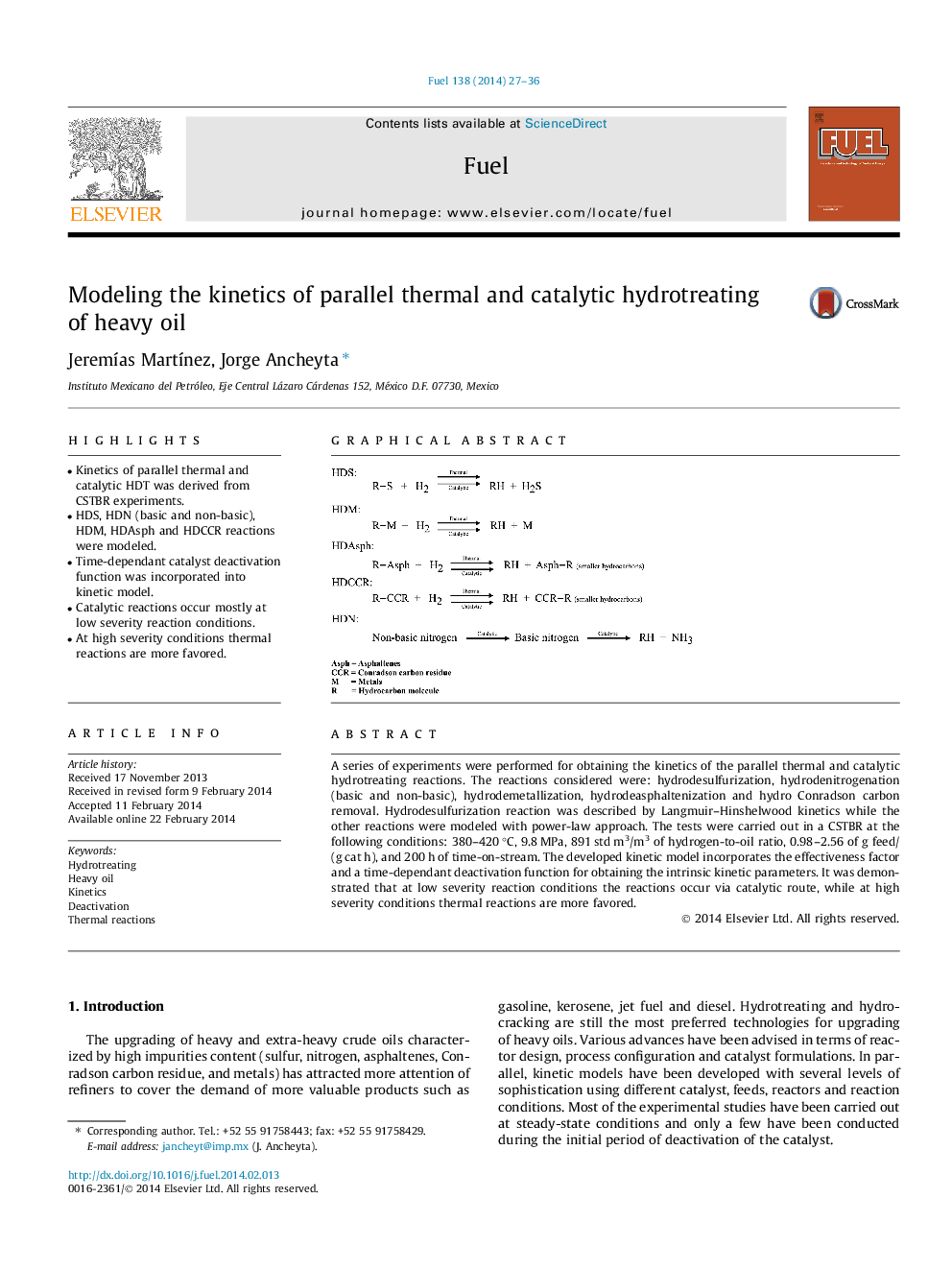
•Kinetics of parallel thermal and catalytic HDT was derived from CSTBR experiments.•HDS, HDN (basic and non-basic), HDM, HDAsph and HDCCR reactions were modeled.•Time-dependant catalyst deactivation function was incorporated into kinetic model.•Catalytic reactions occur mostly at low severity reaction conditions.•At high severity conditions thermal reactions are more favored.
A series of experiments were performed for obtaining the kinetics of the parallel thermal and catalytic hydrotreating reactions. The reactions considered were: hydrodesulfurization, hydrodenitrogenation (basic and non-basic), hydrodemetallization, hydrodeasphaltenization and hydro Conradson carbon removal. Hydrodesulfurization reaction was described by Langmuir–Hinshelwood kinetics while the other reactions were modeled with power-law approach. The tests were carried out in a CSTBR at the following conditions: 380–420 °C, 9.8 MPa, 891 std m3/m3 of hydrogen-to-oil ratio, 0.98–2.56 of g feed/(g cat h), and 200 h of time-on-stream. The developed kinetic model incorporates the effectiveness factor and a time-dependant deactivation function for obtaining the intrinsic kinetic parameters. It was demonstrated that at low severity reaction conditions the reactions occur via catalytic route, while at high severity conditions thermal reactions are more favored.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide