| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 206408 | Fuel | 2012 | 10 Pages |
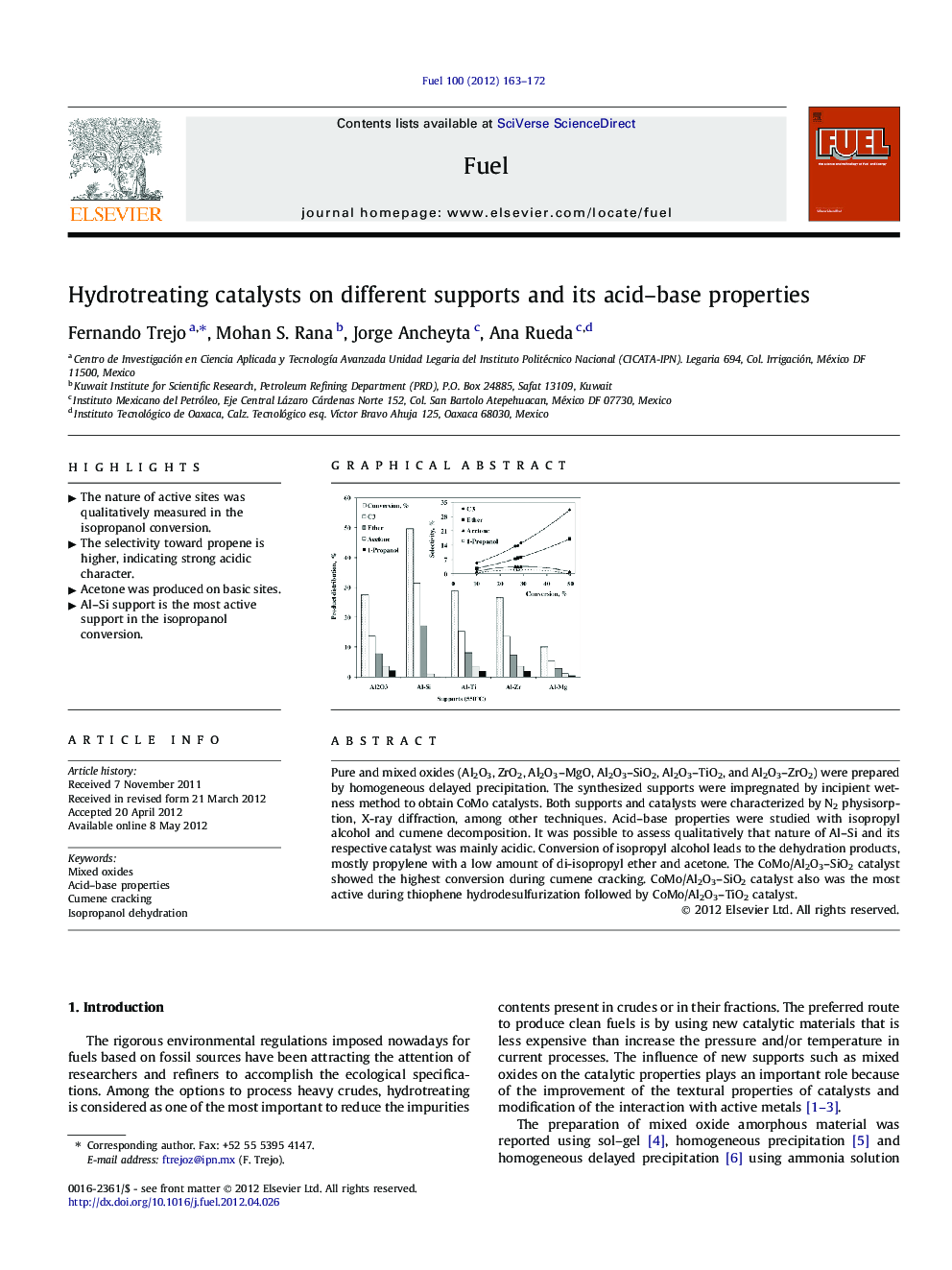
Pure and mixed oxides (Al2O3, ZrO2, Al2O3–MgO, Al2O3–SiO2, Al2O3–TiO2, and Al2O3–ZrO2) were prepared by homogeneous delayed precipitation. The synthesized supports were impregnated by incipient wetness method to obtain CoMo catalysts. Both supports and catalysts were characterized by N2 physisorption, X-ray diffraction, among other techniques. Acid–base properties were studied with isopropyl alcohol and cumene decomposition. It was possible to assess qualitatively that nature of Al–Si and its respective catalyst was mainly acidic. Conversion of isopropyl alcohol leads to the dehydration products, mostly propylene with a low amount of di-isopropyl ether and acetone. The CoMo/Al2O3–SiO2 catalyst showed the highest conversion during cumene cracking. CoMo/Al2O3–SiO2 catalyst also was the most active during thiophene hydrodesulfurization followed by CoMo/Al2O3–TiO2 catalyst.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► The nature of active sites was qualitatively measured in the isopropanol conversion. ► The selectivity toward propene is higher, indicating strong acidic character. ► Acetone was produced on basic sites. ► Al–Si support is the most active support in the isopropanol conversion.