| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2077368 | Cell Stem Cell | 2015 | 16 Pages |
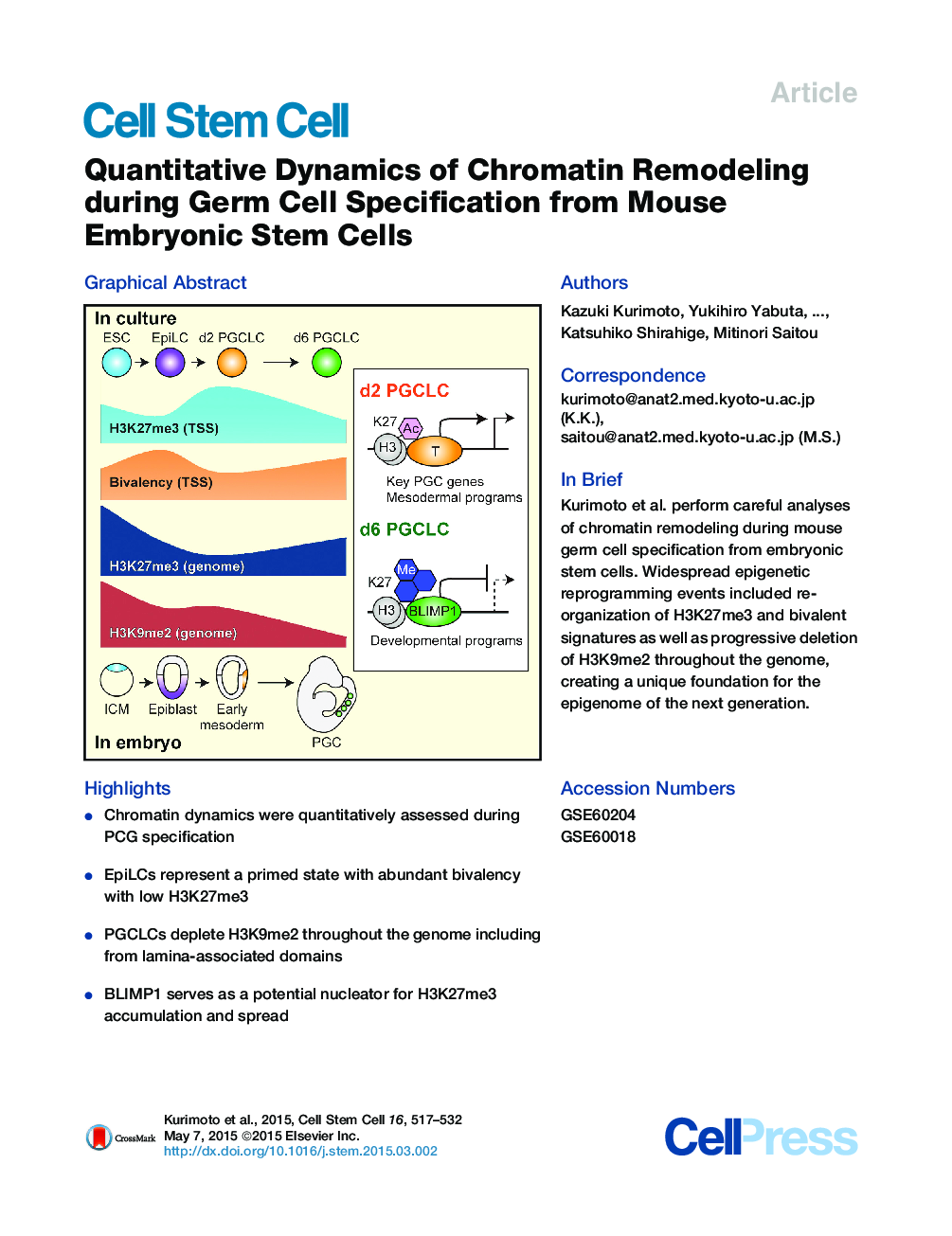
•Chromatin dynamics were quantitatively assessed during PCG specification•EpiLCs represent a primed state with abundant bivalency with low H3K27me3•PGCLCs deplete H3K9me2 throughout the genome including from lamina-associated domains•BLIMP1 serves as a potential nucleator for H3K27me3 accumulation and spread
SummaryGerm cell specification is accompanied by epigenetic remodeling, the scale and specificity of which are unclear. Here, we quantitatively delineate chromatin dynamics during induction of mouse embryonic stem cells (ESCs) to epiblast-like cells (EpiLCs) and from there into primordial germ cell-like cells (PGCLCs), revealing large-scale reorganization of chromatin signatures including H3K27me3 and H3K9me2 patterns. EpiLCs contain abundant bivalent gene promoters characterized by low H3K27me3, indicating a state primed for differentiation. PGCLCs initially lose H3K4me3 from many bivalent genes but subsequently regain this mark with concomitant upregulation of H3K27me3, particularly at developmental regulatory genes. PGCLCs progressively lose H3K9me2, including at lamina-associated perinuclear heterochromatin, resulting in changes in nuclear architecture. T recruits H3K27ac to activate BLIMP1 and early mesodermal programs during PGCLC specification, which is followed by BLIMP1-mediated repression of a broad range of targets, possibly through recruitment and spreading of H3K27me3. These findings provide a foundation for reconstructing regulatory networks of the germline epigenome.
Graphical AbstractFigure optionsDownload full-size imageDownload high-quality image (178 K)Download as PowerPoint slide