| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 231818 | The Journal of Supercritical Fluids | 2008 | 7 Pages |
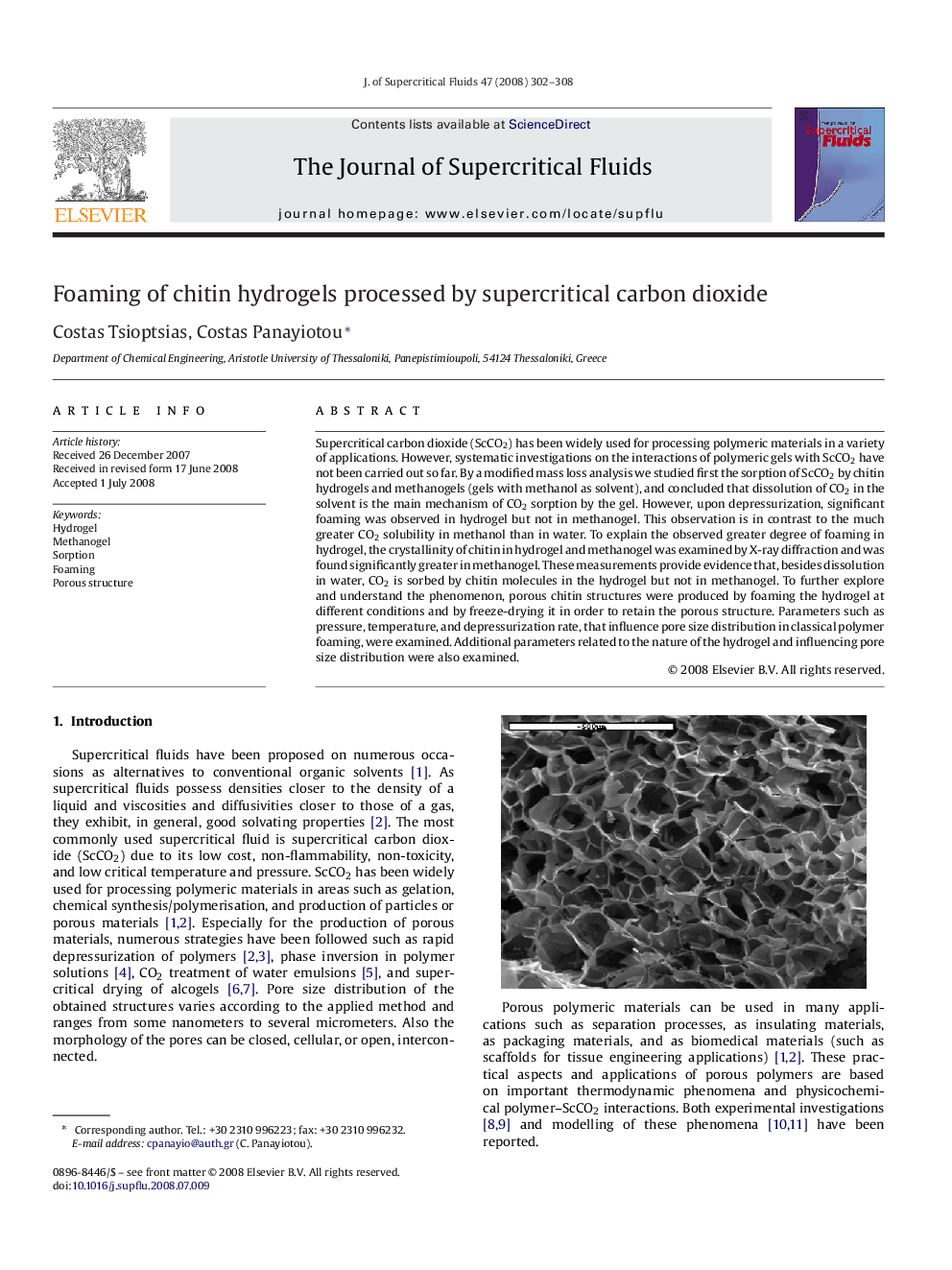
Supercritical carbon dioxide (ScCO2) has been widely used for processing polymeric materials in a variety of applications. However, systematic investigations on the interactions of polymeric gels with ScCO2 have not been carried out so far. By a modified mass loss analysis we studied first the sorption of ScCO2 by chitin hydrogels and methanogels (gels with methanol as solvent), and concluded that dissolution of CO2 in the solvent is the main mechanism of CO2 sorption by the gel. However, upon depressurization, significant foaming was observed in hydrogel but not in methanogel. This observation is in contrast to the much greater CO2 solubility in methanol than in water. To explain the observed greater degree of foaming in hydrogel, the crystallinity of chitin in hydrogel and methanogel was examined by X-ray diffraction and was found significantly greater in methanogel. These measurements provide evidence that, besides dissolution in water, CO2 is sorbed by chitin molecules in the hydrogel but not in methanogel. To further explore and understand the phenomenon, porous chitin structures were produced by foaming the hydrogel at different conditions and by freeze-drying it in order to retain the porous structure. Parameters such as pressure, temperature, and depressurization rate, that influence pore size distribution in classical polymer foaming, were examined. Additional parameters related to the nature of the hydrogel and influencing pore size distribution were also examined.