| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2743218 | Anaesthesia & Intensive Care Medicine | 2007 | 4 Pages |
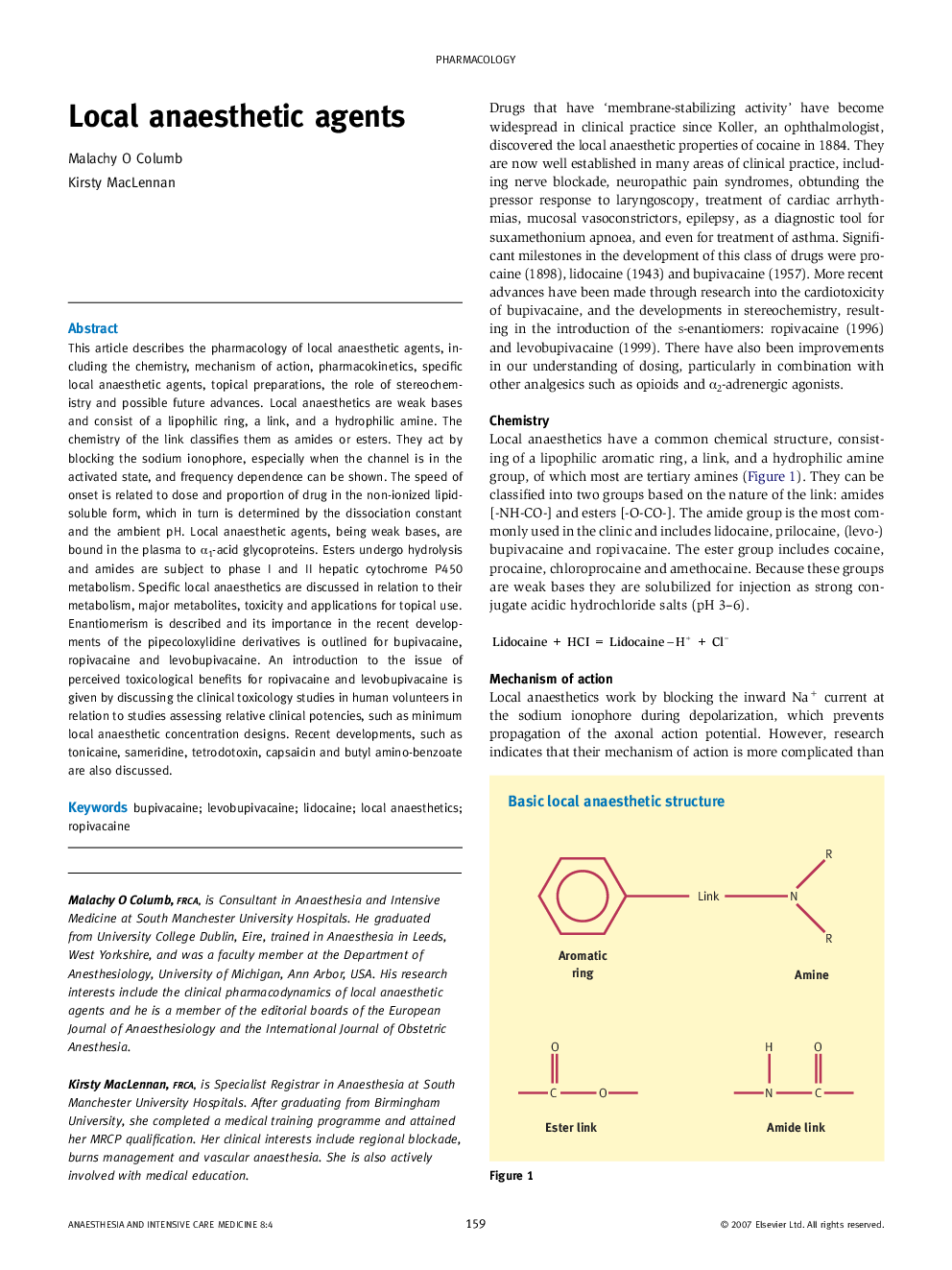
This article describes the pharmacology of local anaesthetic agents, including the chemistry, mechanism of action, pharmacokinetics, specific local anaesthetic agents, topical preparations, the role of stereochemistry and possible future advances. Local anaesthetics are weak bases and consist of a lipophilic ring, a link, and a hydrophilic amine. The chemistry of the link classifies them as amides or esters. They act by blocking the sodium ionophore, especially when the channel is in the activated state, and frequency dependence can be shown. The speed of onset is related to dose and proportion of drug in the non-ionized lipid-soluble form, which in turn is determined by the dissociation constant and the ambient pH. Local anaesthetic agents, being weak bases, are bound in the plasma to α1-acid glycoproteins. Esters undergo hydrolysis and amides are subject to phase I and II hepatic cytochrome P450 metabolism. Specific local anaesthetics are discussed in relation to their metabolism, major metabolites, toxicity and applications for topical use. Enantiomerism is described and its importance in the recent developments of the pipecoloxylidine derivatives is outlined for bupivacaine, ropivacaine and levobupivacaine. An introduction to the issue of perceived toxicological benefits for ropivacaine and levobupivacaine is given by discussing the clinical toxicology studies in human volunteers in relation to studies assessing relative clinical potencies, such as minimum local anaesthetic concentration designs. Recent developments, such as tonicaine, sameridine, tetrodotoxin, capsaicin and butyl amino-benzoate are also discussed.