| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2792464 | Cell Metabolism | 2015 | 12 Pages |
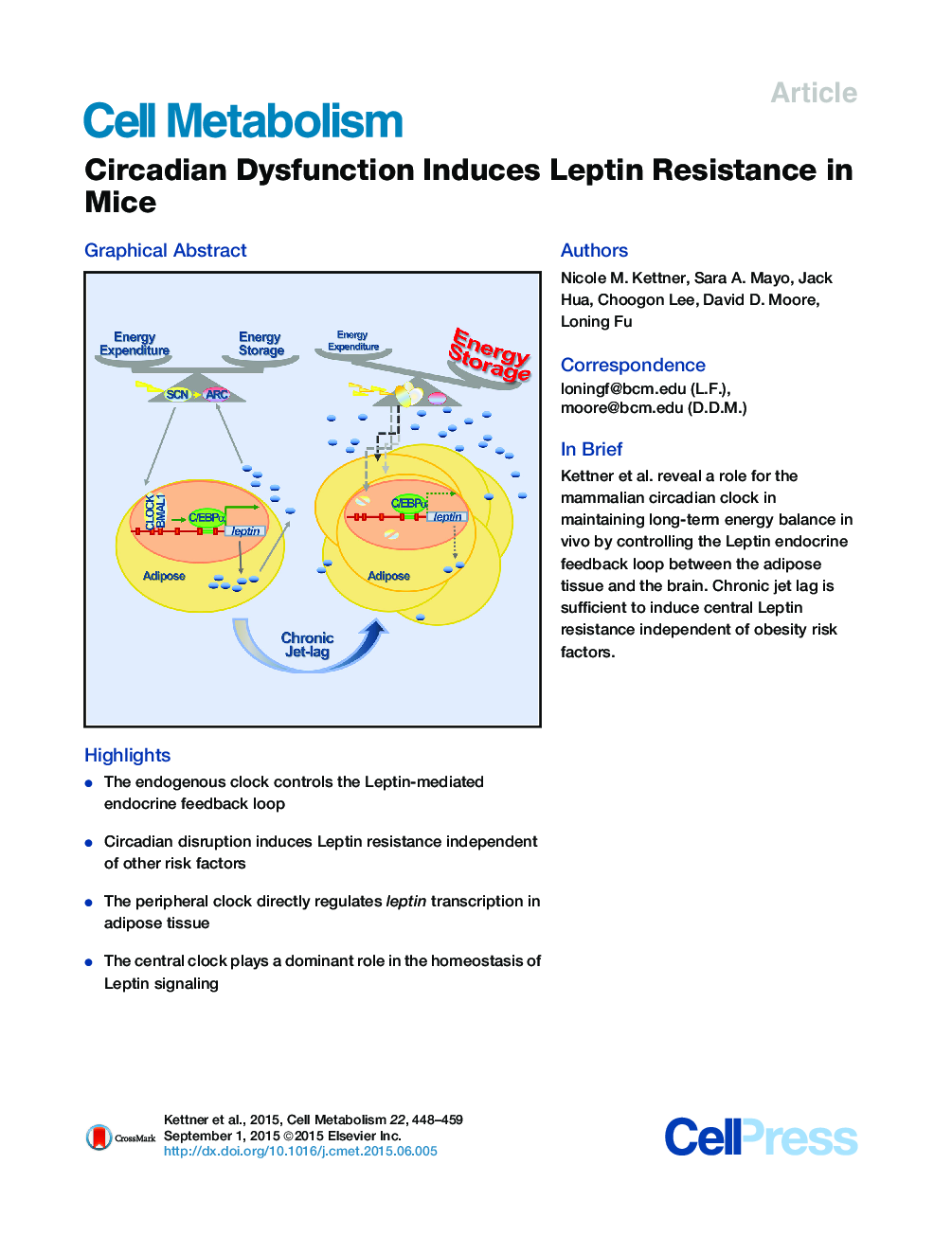
•The endogenous clock controls the Leptin-mediated endocrine feedback loop•Circadian disruption induces Leptin resistance independent of other risk factors•The peripheral clock directly regulates leptin transcription in adipose tissue•The central clock plays a dominant role in the homeostasis of Leptin signaling
SummaryCircadian disruption is associated with obesity, implicating the central clock in body weight control. Our comprehensive screen of wild-type and three circadian mutant mouse models, with or without chronic jet lag, shows that distinct genetic and physiologic interventions differentially disrupt overall energy homeostasis and Leptin signaling. We found that BMAL1/CLOCK generates circadian rhythm of C/EBPα-mediated leptin transcription in adipose. Per and Cry mutant mice show similar disruption of peripheral clock and deregulation of leptin in fat, but opposite body weight and composition phenotypes that correlate with their distinct patterns of POMC neuron deregulation in the arcuate nucleus. Chronic jet lag is sufficient to disrupt the endogenous adipose clock and also induce central Leptin resistance in wild-type mice. Thus, coupling of the central and peripheral clocks controls Leptin endocrine feedback homeostasis. We propose that Leptin resistance, a hallmark of obesity in humans, plays a key role in circadian dysfunction-induced obesity and metabolic syndromes.
Graphical AbstractFigure optionsDownload full-size imageDownload high-quality image (183 K)Download as PowerPoint slide