| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2792557 | Cell Metabolism | 2015 | 10 Pages |
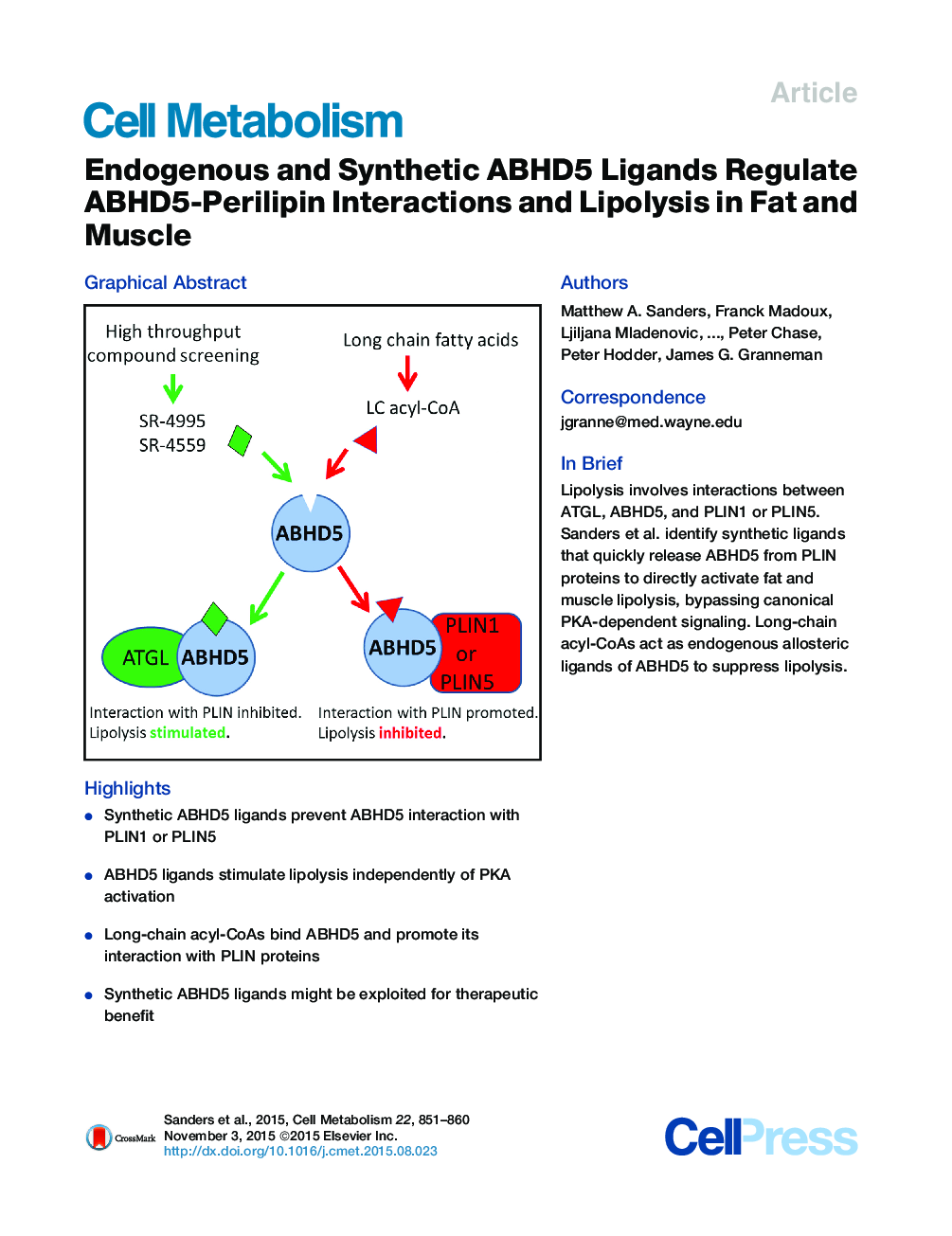
•Synthetic ABHD5 ligands prevent ABHD5 interaction with PLIN1 or PLIN5•ABHD5 ligands stimulate lipolysis independently of PKA activation•Long-chain acyl-CoAs bind ABHD5 and promote its interaction with PLIN proteins•Synthetic ABHD5 ligands might be exploited for therapeutic benefit
SummaryFat and muscle lipolysis involves functional interactions of adipose triglyceride lipase (ATGL), α-β hydrolase domain-containing protein 5 (ABHD5), and tissue-specific perilipins 1 and 5 (PLIN1 and PLIN5). ABHD5 potently activates ATGL, but this lipase-promoting activity is suppressed when ABHD5 is bound to PLIN proteins on lipid droplets. In adipocytes, protein kinase A (PKA) phosphorylation of PLIN1 rapidly releases ABHD5 to activate ATGL, but mechanisms for rapid regulation of PLIN5-ABHD5 interaction in muscle are unknown. Here, we identify synthetic ligands that release ABHD5 from PLIN1 or PLIN5 without PKA activation and rapidly activate adipocyte and muscle lipolysis. Molecular imaging and affinity probe labeling demonstrated that ABHD5 is directly targeted by these synthetic ligands and additionally revealed that ABHD5-PLIN interactions are regulated by endogenous ligands, including long-chain acyl-CoA. Our results reveal a new locus of lipolysis control and suggest ABHD5 ligands might be developed into novel therapeutics that directly promote fat catabolism.
Graphical AbstractFigure optionsDownload full-size imageDownload high-quality image (148 K)Download as PowerPoint slide