| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4407291 | Chemosphere | 2016 | 8 Pages |
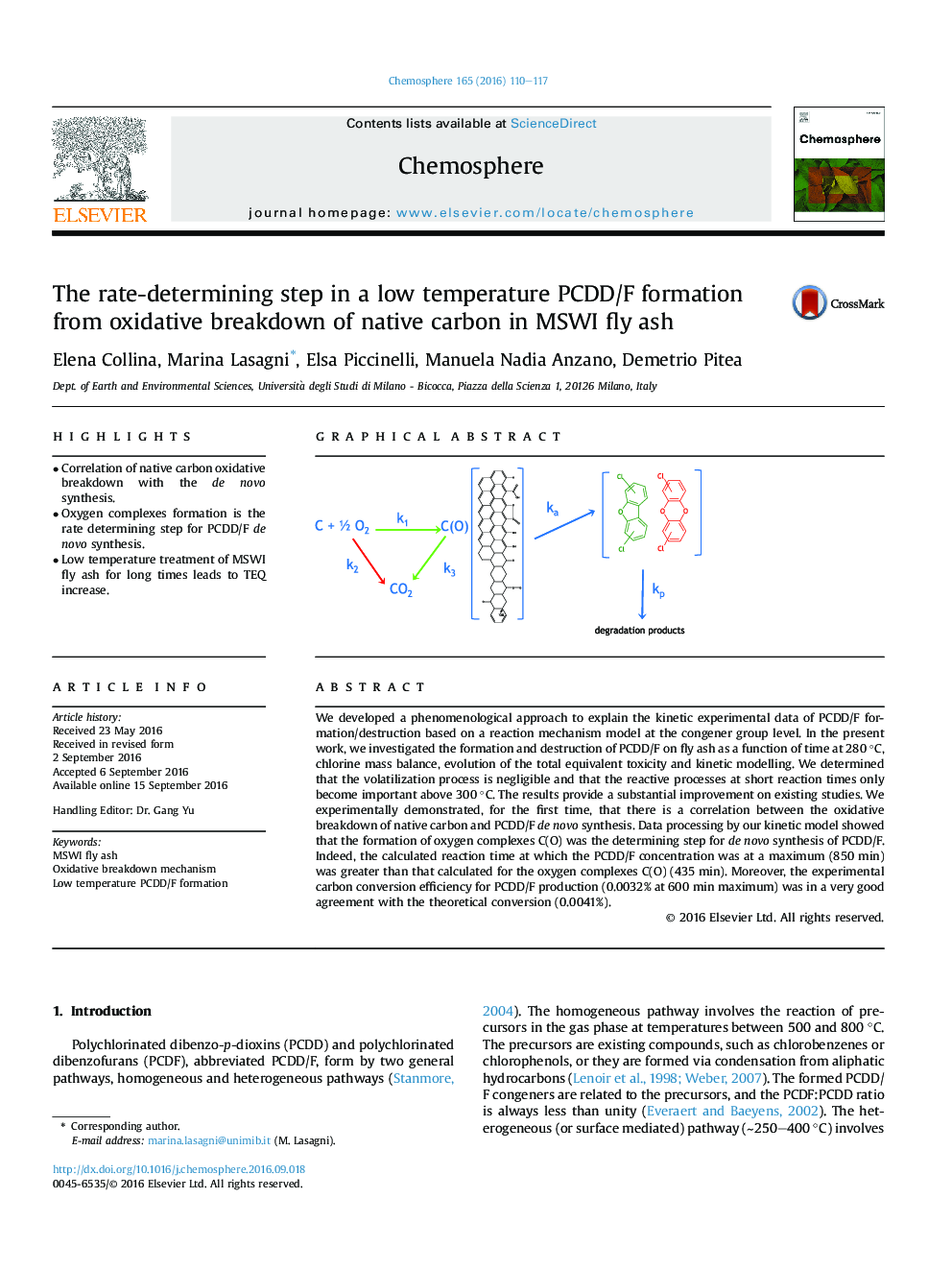
•Correlation of native carbon oxidative breakdown with the de novo synthesis.•Oxygen complexes formation is the rate determining step for PCDD/F de novo synthesis.•Low temperature treatment of MSWI fly ash for long times leads to TEQ increase.
We developed a phenomenological approach to explain the kinetic experimental data of PCDD/F formation/destruction based on a reaction mechanism model at the congener group level. In the present work, we investigated the formation and destruction of PCDD/F on fly ash as a function of time at 280 °C, chlorine mass balance, evolution of the total equivalent toxicity and kinetic modelling. We determined that the volatilization process is negligible and that the reactive processes at short reaction times only become important above 300 °C. The results provide a substantial improvement on existing studies. We experimentally demonstrated, for the first time, that there is a correlation between the oxidative breakdown of native carbon and PCDD/F de novo synthesis. Data processing by our kinetic model showed that the formation of oxygen complexes C(O) was the determining step for de novo synthesis of PCDD/F. Indeed, the calculated reaction time at which the PCDD/F concentration was at a maximum (850 min) was greater than that calculated for the oxygen complexes C(O) (435 min). Moreover, the experimental carbon conversion efficiency for PCDD/F production (0.0032% at 600 min maximum) was in a very good agreement with the theoretical conversion (0.0041%).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide