| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4407339 | Chemosphere | 2016 | 10 Pages |
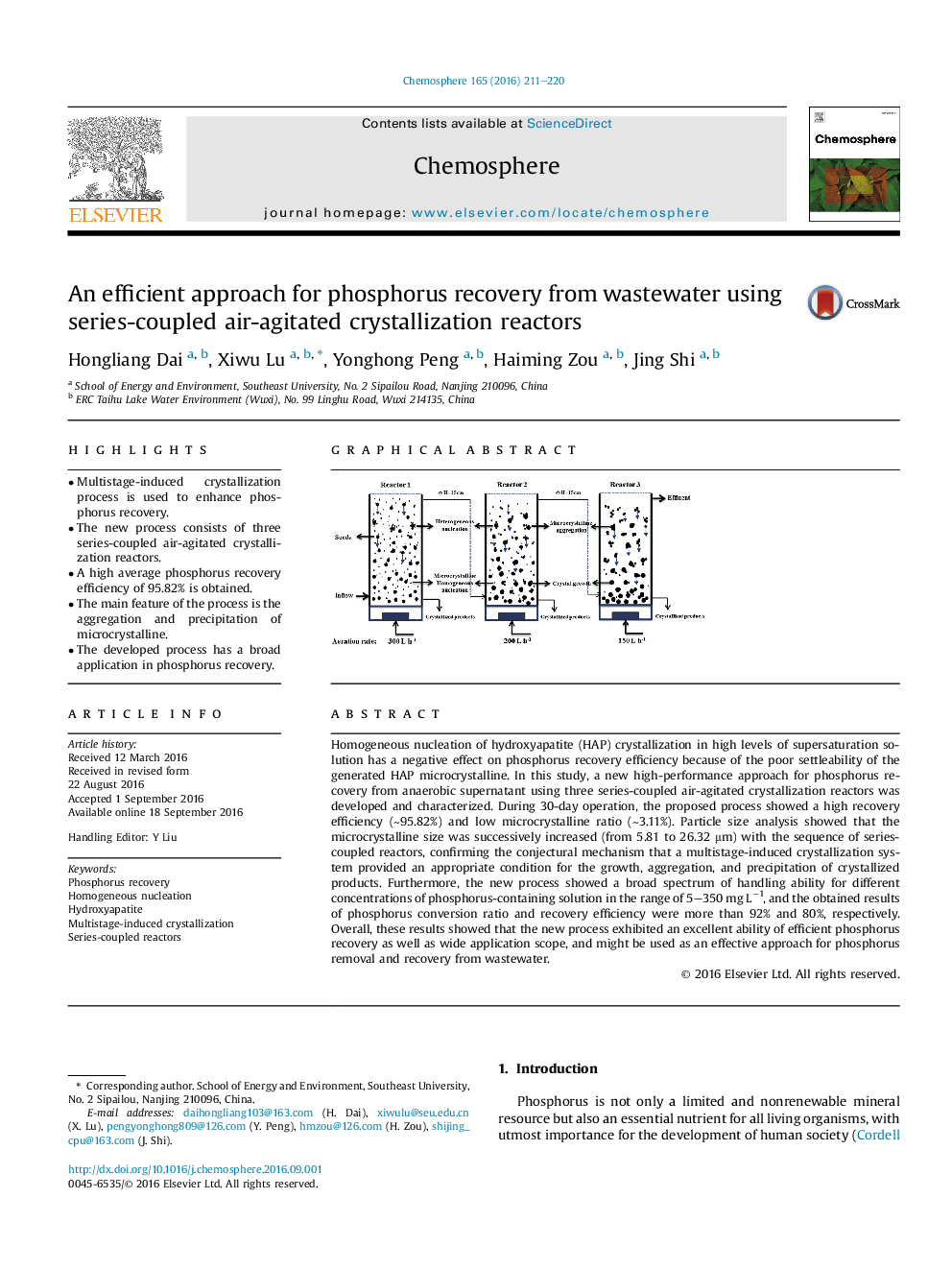
•Multistage-induced crystallization process is used to enhance phosphorus recovery.•The new process consists of three series-coupled air-agitated crystallization reactors.•A high average phosphorus recovery efficiency of 95.82% is obtained.•The main feature of the process is the aggregation and precipitation of microcrystalline.•The developed process has a broad application in phosphorus recovery.
Homogeneous nucleation of hydroxyapatite (HAP) crystallization in high levels of supersaturation solution has a negative effect on phosphorus recovery efficiency because of the poor settleability of the generated HAP microcrystalline. In this study, a new high-performance approach for phosphorus recovery from anaerobic supernatant using three series-coupled air-agitated crystallization reactors was developed and characterized. During 30-day operation, the proposed process showed a high recovery efficiency (∼95.82%) and low microcrystalline ratio (∼3.11%). Particle size analysis showed that the microcrystalline size was successively increased (from 5.81 to 26.32 μm) with the sequence of series-coupled reactors, confirming the conjectural mechanism that a multistage-induced crystallization system provided an appropriate condition for the growth, aggregation, and precipitation of crystallized products. Furthermore, the new process showed a broad spectrum of handling ability for different concentrations of phosphorus-containing solution in the range of 5–350 mg L−1, and the obtained results of phosphorus conversion ratio and recovery efficiency were more than 92% and 80%, respectively. Overall, these results showed that the new process exhibited an excellent ability of efficient phosphorus recovery as well as wide application scope, and might be used as an effective approach for phosphorus removal and recovery from wastewater.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide