| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4407568 | Chemosphere | 2016 | 9 Pages |
•Oriented attachment crystal growth of magnetite and goethite favoured by the presence of aluminium.•Increased capacity for As retention by nanostructured Al-magnetite relative to Al-free magnetite.•Negative correlation between Al and As in magnetite for Al/(Al + Fe) higher than 3–5%.•Arsenic trapped into Al-magnetite mesocrystals growing by oriented aggregation.•Trace amounts of As detected in As-Al-Fe co-precipitates by high-resolution electron microscopy techniques.
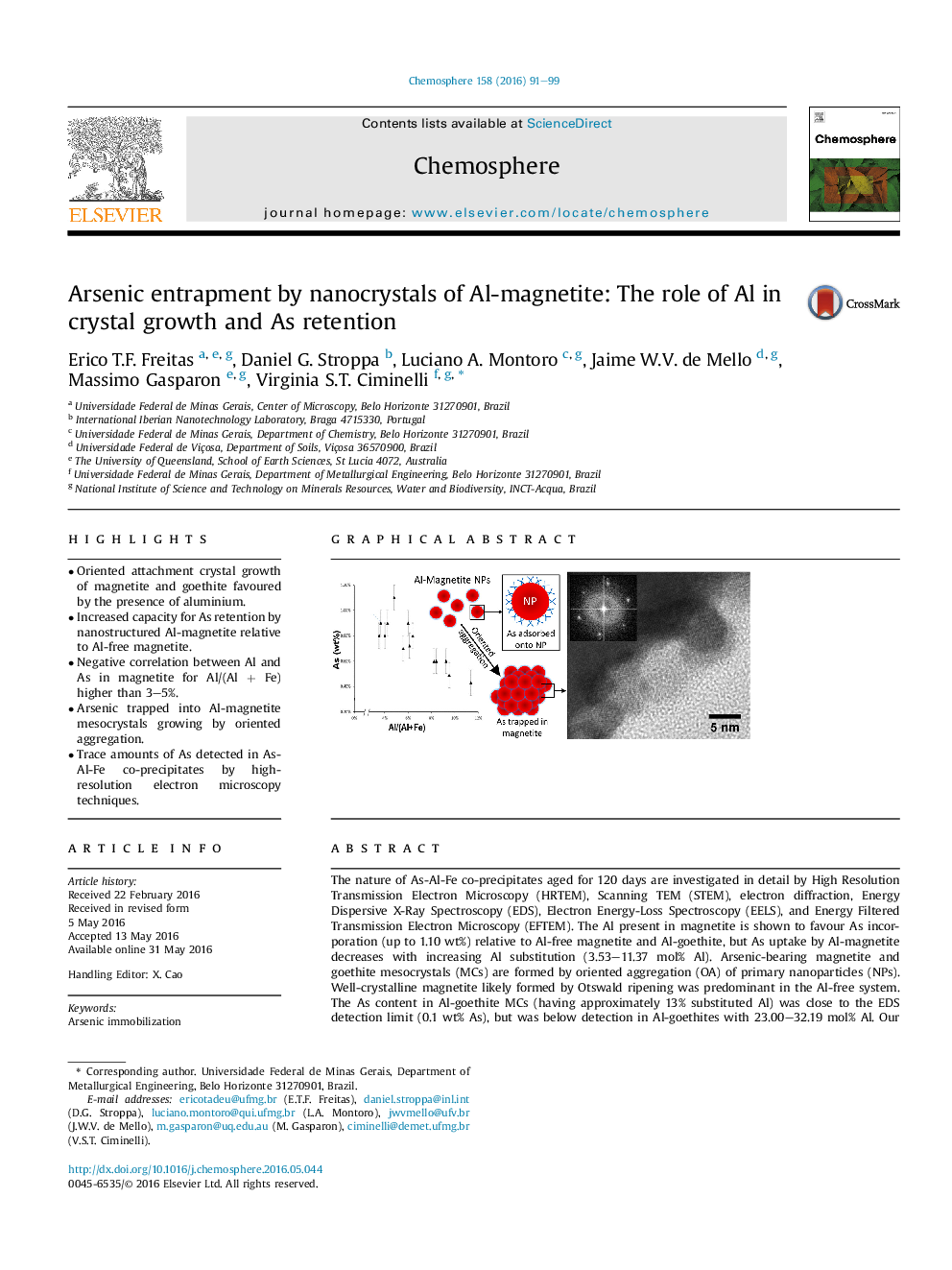
The nature of As-Al-Fe co-precipitates aged for 120 days are investigated in detail by High Resolution Transmission Electron Microscopy (HRTEM), Scanning TEM (STEM), electron diffraction, Energy Dispersive X-Ray Spectroscopy (EDS), Electron Energy-Loss Spectroscopy (EELS), and Energy Filtered Transmission Electron Microscopy (EFTEM). The Al present in magnetite is shown to favour As incorporation (up to 1.10 wt%) relative to Al-free magnetite and Al-goethite, but As uptake by Al-magnetite decreases with increasing Al substitution (3.53–11.37 mol% Al). Arsenic-bearing magnetite and goethite mesocrystals (MCs) are formed by oriented aggregation (OA) of primary nanoparticles (NPs). Well-crystalline magnetite likely formed by Otswald ripening was predominant in the Al-free system. The As content in Al-goethite MCs (having approximately 13% substituted Al) was close to the EDS detection limit (0.1 wt% As), but was below detection in Al-goethites with 23.00–32.19 mol% Al. Our results show for the first time the capacity of Al-magnetite to incorporate more As than Al-free magnetite, and the role of Al in favouring OA-based crystal growth under the experimental conditions, and therefore As retention in the formed MCs. The proposed mechanism of As incorporation involves adsorption of As onto the newly formed NPs. Arsenic is then trapped in the MCs as they grow by self-assembly OA upon attachment of the NPs. We conclude that Al may diffuse to the crystal faces with high surface energy to reduce the total energy of the system during the attachment events, thus favouring the oriented aggregation.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide