| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4407617 | Chemosphere | 2016 | 9 Pages |
•Pd, Rh, and Rh-Pd nanoparticles are deposited on Aluminum powder via spontaneous redox reactions in aqueous solutions at room temperature.•Aluminum supported metals, an easy preparation catalysts, used for aqueous phase polybrominated diphenyl ethers in sc-CO2.•The conditions for hydrobromination are green, hydrodebrominating 4- and 4,4′-bromodiphenyl ethers into diphenyl ether in sc-CO2 below 100 °C.•Hydrogenolysis of diphenyl ether producing cyclohexanol and cyclohexane is the minor reaction route.
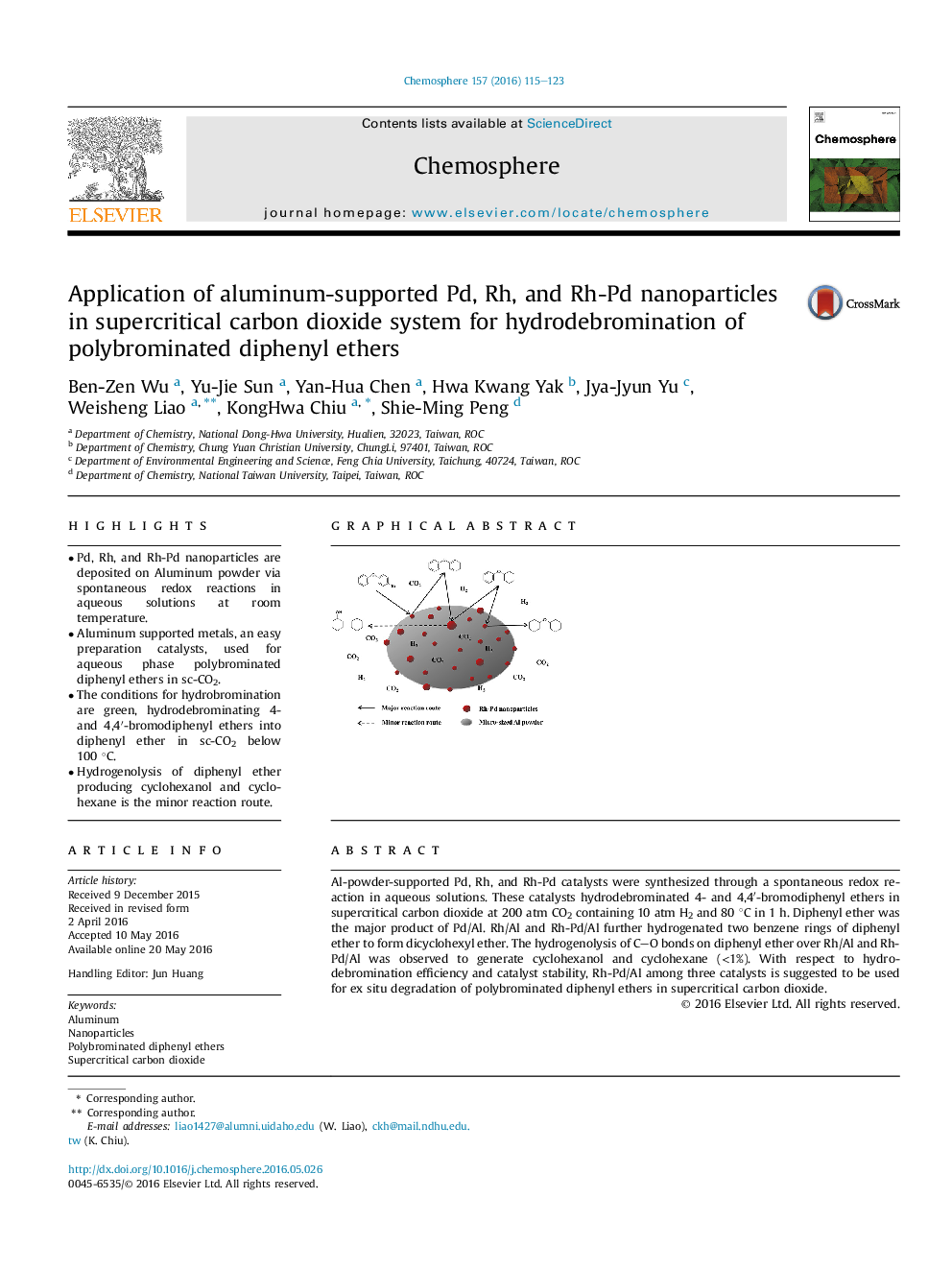
Al-powder-supported Pd, Rh, and Rh-Pd catalysts were synthesized through a spontaneous redox reaction in aqueous solutions. These catalysts hydrodebrominated 4- and 4,4′-bromodiphenyl ethers in supercritical carbon dioxide at 200 atm CO2 containing 10 atm H2 and 80 °C in 1 h. Diphenyl ether was the major product of Pd/Al. Rh/Al and Rh-Pd/Al further hydrogenated two benzene rings of diphenyl ether to form dicyclohexyl ether. The hydrogenolysis of CO bonds on diphenyl ether over Rh/Al and Rh-Pd/Al was observed to generate cyclohexanol and cyclohexane (<1%). With respect to hydrodebromination efficiency and catalyst stability, Rh-Pd/Al among three catalysts is suggested to be used for ex situ degradation of polybrominated diphenyl ethers in supercritical carbon dioxide.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide