| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4407821 | Chemosphere | 2016 | 6 Pages |
•Deprotonation of humic acids is required to obtain stable complexes with CL-20.•CL-20 binds to deprotonated humic acids through the formation of hydrogen bonds.•CL-20 adsorbed by humic acids is expected to be resistant to redox transformations.•Solvation promotes redox transformation of CL-20, an important industrial explosive.
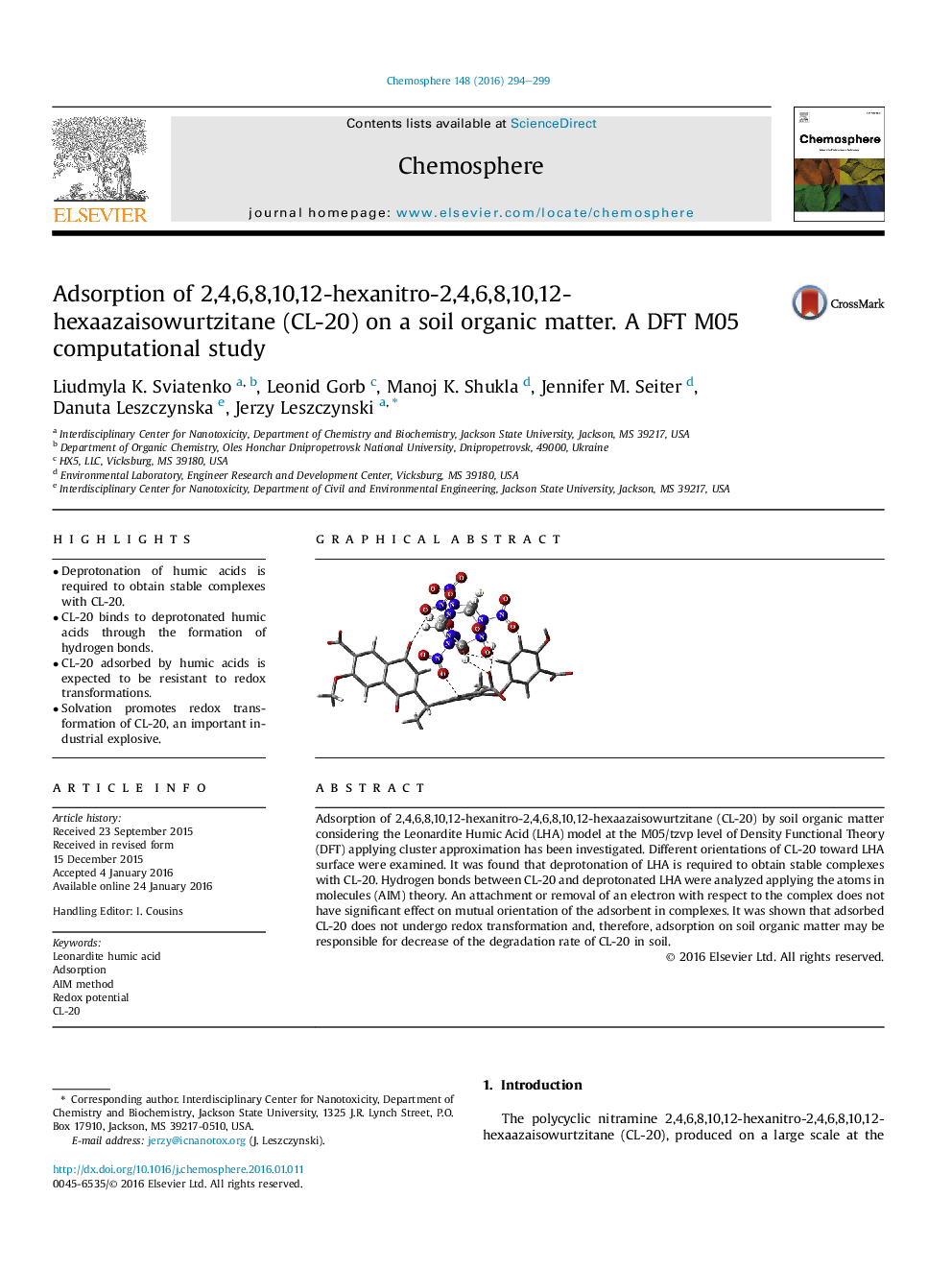
Adsorption of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) by soil organic matter considering the Leonardite Humic Acid (LHA) model at the M05/tzvp level of Density Functional Theory (DFT) applying cluster approximation has been investigated. Different orientations of CL-20 toward LHA surface were examined. It was found that deprotonation of LHA is required to obtain stable complexes with CL-20. Hydrogen bonds between CL-20 and deprotonated LHA were analyzed applying the atoms in molecules (AIM) theory. An attachment or removal of an electron with respect to the complex does not have significant effect on mutual orientation of the adsorbent in complexes. It was shown that adsorbed CL-20 does not undergo redox transformation and, therefore, adsorption on soil organic matter may be responsible for decrease of the degradation rate of CL-20 in soil.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide