| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4408281 | Chemosphere | 2015 | 8 Pages |
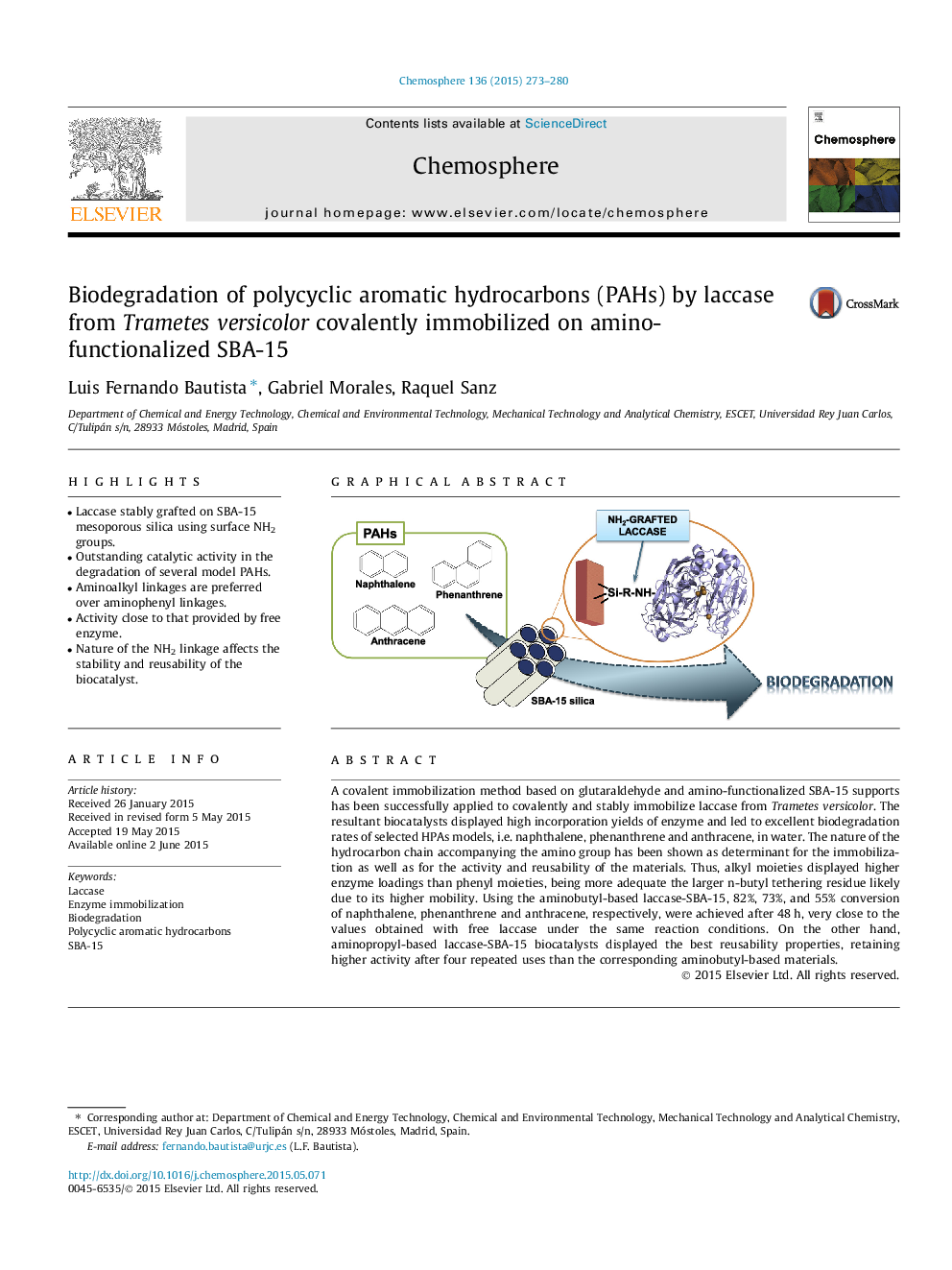
•Laccase stably grafted on SBA-15 mesoporous silica using surface NH2 groups.•Outstanding catalytic activity in the degradation of several model PAHs.•Aminoalkyl linkages are preferred over aminophenyl linkages.•Activity close to that provided by free enzyme.•Nature of the NH2 linkage affects the stability and reusability of the biocatalyst.
A covalent immobilization method based on glutaraldehyde and amino-functionalized SBA-15 supports has been successfully applied to covalently and stably immobilize laccase from Trametes versicolor. The resultant biocatalysts displayed high incorporation yields of enzyme and led to excellent biodegradation rates of selected HPAs models, i.e. naphthalene, phenanthrene and anthracene, in water. The nature of the hydrocarbon chain accompanying the amino group has been shown as determinant for the immobilization as well as for the activity and reusability of the materials. Thus, alkyl moieties displayed higher enzyme loadings than phenyl moieties, being more adequate the larger n-butyl tethering residue likely due to its higher mobility. Using the aminobutyl-based laccase-SBA-15, 82%, 73%, and 55% conversion of naphthalene, phenanthrene and anthracene, respectively, were achieved after 48 h, very close to the values obtained with free laccase under the same reaction conditions. On the other hand, aminopropyl-based laccase-SBA-15 biocatalysts displayed the best reusability properties, retaining higher activity after four repeated uses than the corresponding aminobutyl-based materials.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide