| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4408386 | Chemosphere | 2015 | 9 Pages |
•Significant enantioselective toxicity was found for tebuconazole and myclobutanil.•Enantioselective degradation of two compounds was observed in 7 different soils.•The enantioselectivity of degradation showed a relationship with soil properties.•Both aerobic and anaerobic conditions have the same direction of enantioselectivity.•Chiral tebuconazole and myclobutanil were configurationally stable in soils.
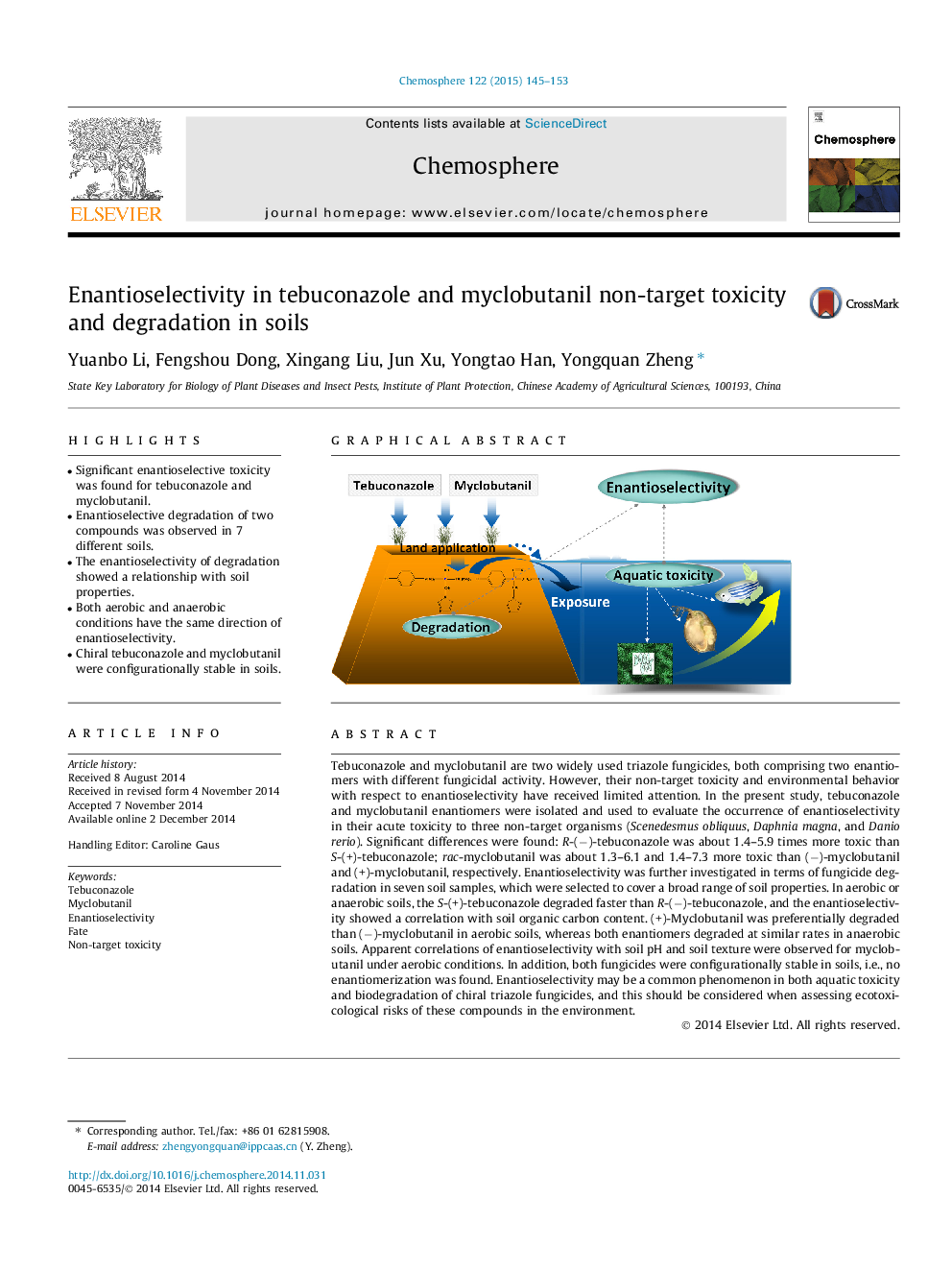
Tebuconazole and myclobutanil are two widely used triazole fungicides, both comprising two enantiomers with different fungicidal activity. However, their non-target toxicity and environmental behavior with respect to enantioselectivity have received limited attention. In the present study, tebuconazole and myclobutanil enantiomers were isolated and used to evaluate the occurrence of enantioselectivity in their acute toxicity to three non-target organisms (Scenedesmus obliquus, Daphnia magna, and Danio rerio). Significant differences were found: R-(−)-tebuconazole was about 1.4–5.9 times more toxic than S-(+)-tebuconazole; rac-myclobutanil was about 1.3–6.1 and 1.4–7.3 more toxic than (−)-myclobutanil and (+)-myclobutanil, respectively. Enantioselectivity was further investigated in terms of fungicide degradation in seven soil samples, which were selected to cover a broad range of soil properties. In aerobic or anaerobic soils, the S-(+)-tebuconazole degraded faster than R-(−)-tebuconazole, and the enantioselectivity showed a correlation with soil organic carbon content. (+)-Myclobutanil was preferentially degraded than (−)-myclobutanil in aerobic soils, whereas both enantiomers degraded at similar rates in anaerobic soils. Apparent correlations of enantioselectivity with soil pH and soil texture were observed for myclobutanil under aerobic conditions. In addition, both fungicides were configurationally stable in soils, i.e., no enantiomerization was found. Enantioselectivity may be a common phenomenon in both aquatic toxicity and biodegradation of chiral triazole fungicides, and this should be considered when assessing ecotoxicological risks of these compounds in the environment.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide