| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4408658 | Chemosphere | 2015 | 6 Pages |
•Formation of the radical complexes between Hg(II) and tannic acid was observed.•Catechol was the simplest model compound undergoing the reaction with Hg(II).•Application of high field EPR spectroscopy unveiled components of the g matrix.•DFT computations of the g matrix revealed the structure of formed complexes.
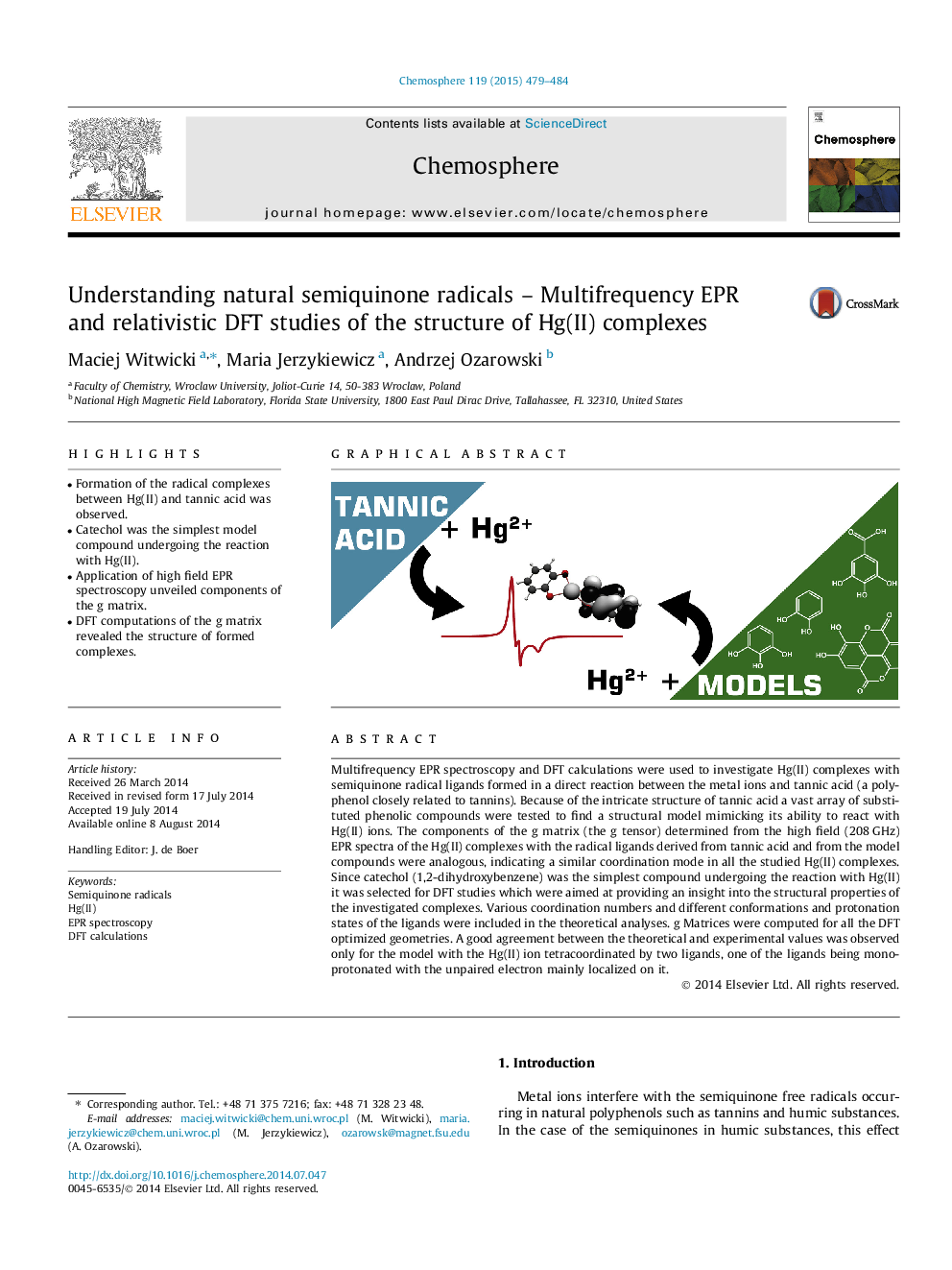
Multifrequency EPR spectroscopy and DFT calculations were used to investigate Hg(II) complexes with semiquinone radical ligands formed in a direct reaction between the metal ions and tannic acid (a polyphenol closely related to tannins). Because of the intricate structure of tannic acid a vast array of substituted phenolic compounds were tested to find a structural model mimicking its ability to react with Hg(II) ions. The components of the g matrix (the g tensor) determined from the high field (208 GHz) EPR spectra of the Hg(II) complexes with the radical ligands derived from tannic acid and from the model compounds were analogous, indicating a similar coordination mode in all the studied Hg(II) complexes. Since catechol (1,2-dihydroxybenzene) was the simplest compound undergoing the reaction with Hg(II) it was selected for DFT studies which were aimed at providing an insight into the structural properties of the investigated complexes. Various coordination numbers and different conformations and protonation states of the ligands were included in the theoretical analyses. g Matrices were computed for all the DFT optimized geometries. A good agreement between the theoretical and experimental values was observed only for the model with the Hg(II) ion tetracoordinated by two ligands, one of the ligands being monoprotonated with the unpaired electron mainly localized on it.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide