| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4428777 | Science of The Total Environment | 2013 | 7 Pages |
•Slow sorption and hysteresis were found in 17α-ethinyl estradiol (EE2) sorption.•EE2 diffusion in micropores rather than organic matter caused slow sorption.•Location difference of hard carbon had a great influence on desorption hysteresis.•Desorption hysteresis decreased with increasing initial EE2 aqueous concentration.•Sorption nonlinearity increased with increasing ratio of black carbon to TOC.
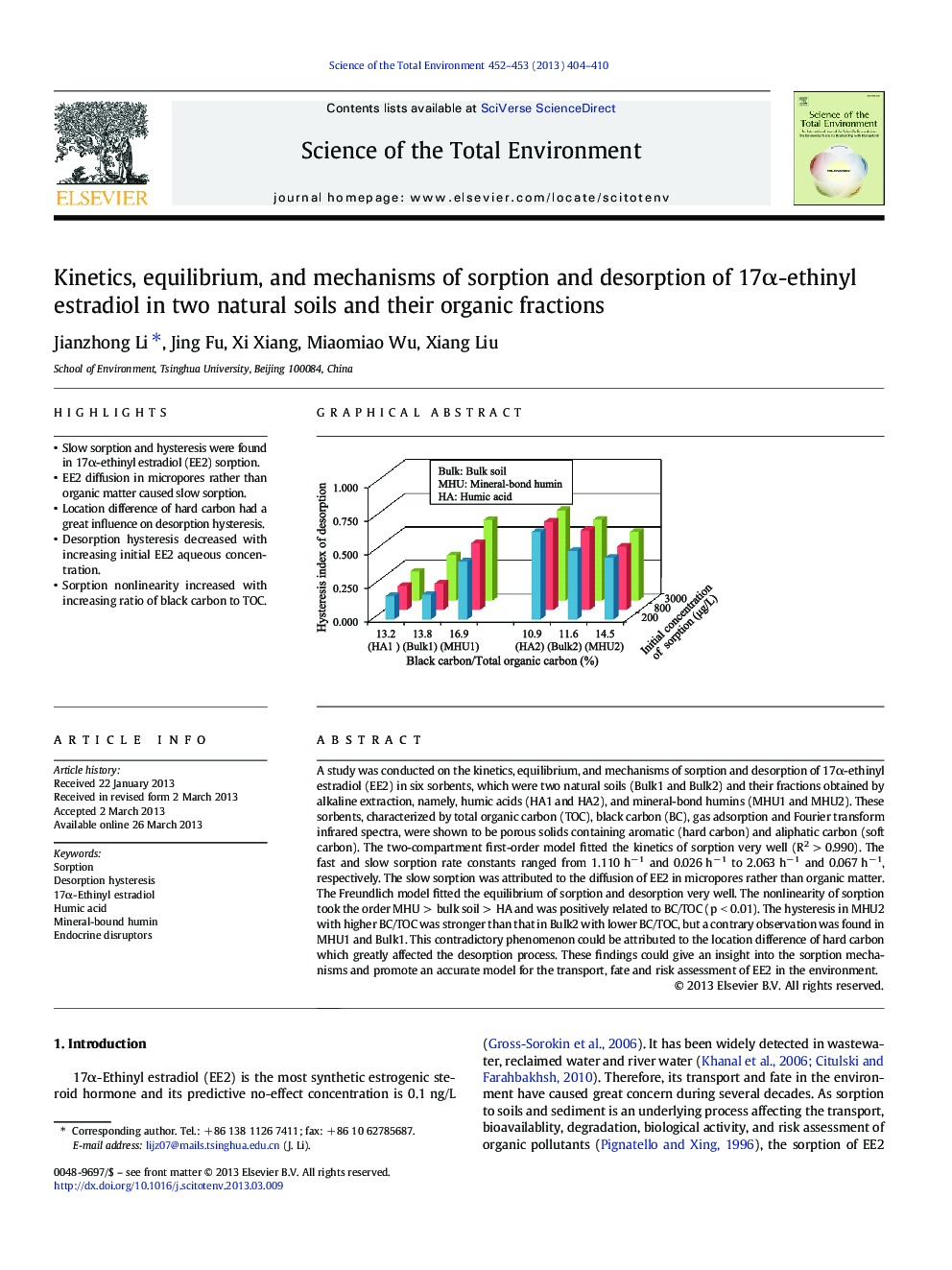
A study was conducted on the kinetics, equilibrium, and mechanisms of sorption and desorption of 17α-ethinyl estradiol (EE2) in six sorbents, which were two natural soils (Bulk1 and Bulk2) and their fractions obtained by alkaline extraction, namely, humic acids (HA1 and HA2), and mineral-bond humins (MHU1 and MHU2). These sorbents, characterized by total organic carbon (TOC), black carbon (BC), gas adsorption and Fourier transform infrared spectra, were shown to be porous solids containing aromatic (hard carbon) and aliphatic carbon (soft carbon). The two-compartment first-order model fitted the kinetics of sorption very well (R2 > 0.990). The fast and slow sorption rate constants ranged from 1.110 h− 1 and 0.026 h− 1 to 2.063 h− 1 and 0.067 h− 1, respectively. The slow sorption was attributed to the diffusion of EE2 in micropores rather than organic matter. The Freundlich model fitted the equilibrium of sorption and desorption very well. The nonlinearity of sorption took the order MHU > bulk soil > HA and was positively related to BC/TOC (p < 0.01). The hysteresis in MHU2 with higher BC/TOC was stronger than that in Bulk2 with lower BC/TOC, but a contrary observation was found in MHU1 and Bulk1. This contradictory phenomenon could be attributed to the location difference of hard carbon which greatly affected the desorption process. These findings could give an insight into the sorption mechanisms and promote an accurate model for the transport, fate and risk assessment of EE2 in the environment.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide