| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4438479 | Atmospheric Environment | 2013 | 8 Pages |
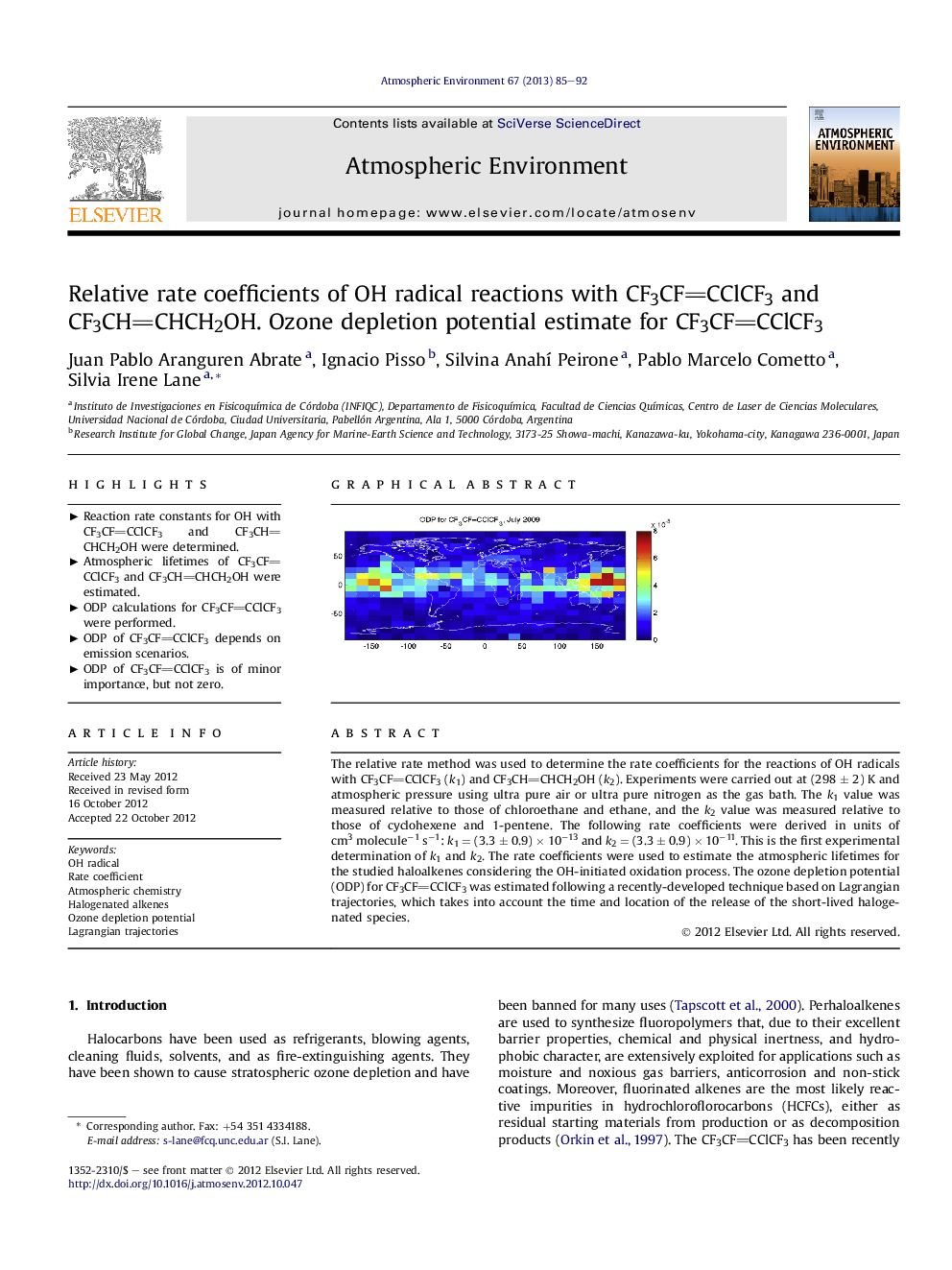
The relative rate method was used to determine the rate coefficients for the reactions of OH radicals with CF3CFCClCF3 (k1) and CF3CHCHCH2OH (k2). Experiments were carried out at (298 ± 2) K and atmospheric pressure using ultra pure air or ultra pure nitrogen as the gas bath. The k1 value was measured relative to those of chloroethane and ethane, and the k2 value was measured relative to those of cyclohexene and 1-pentene. The following rate coefficients were derived in units of cm3 molecule−1 s−1: k1 = (3.3 ± 0.9) × 10−13 and k2 = (3.3 ± 0.9) × 10−11. This is the first experimental determination of k1 and k2. The rate coefficients were used to estimate the atmospheric lifetimes for the studied haloalkenes considering the OH-initiated oxidation process. The ozone depletion potential (ODP) for CF3CFCClCF3 was estimated following a recently-developed technique based on Lagrangian trajectories, which takes into account the time and location of the release of the short-lived halogenated species.
Graphical abstractFigure optionsDownload full-size imageDownload high-quality image (235 K)Download as PowerPoint slideHighlights► Reaction rate constants for OH with CF3CFCClCF3 and CF3CHCHCH2OH were determined. ► Atmospheric lifetimes of CF3CFCClCF3 and CF3CHCHCH2OH were estimated. ► ODP calculations for CF3CFCClCF3 were performed. ► ODP of CF3CFCClCF3 depends on emission scenarios. ► ODP of CF3CFCClCF3 is of minor importance, but not zero.