| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4979246 | Journal of Hazardous Materials | 2018 | 10 Pages |
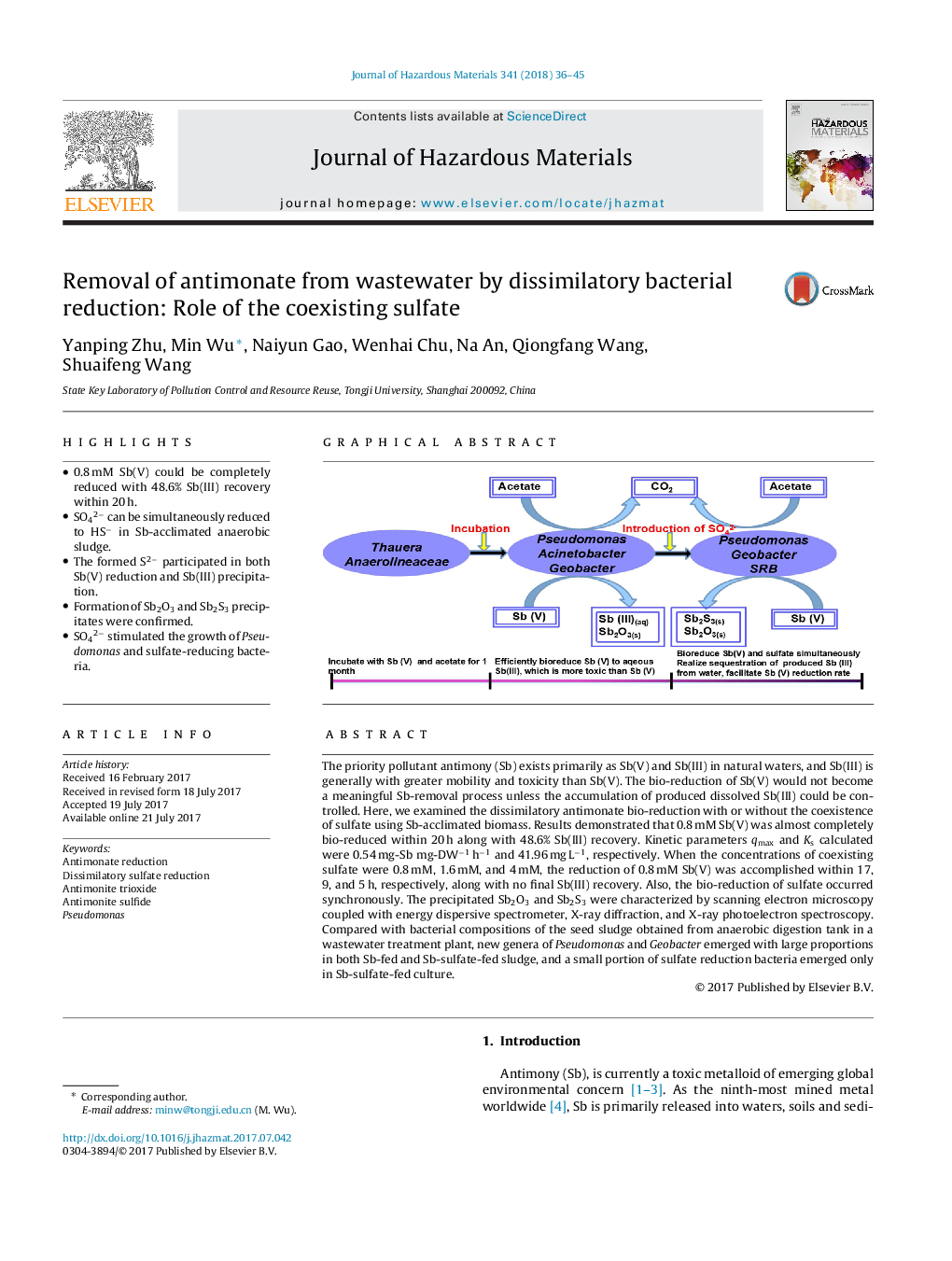
â¢0.8 mM Sb(V) could be completely reduced with 48.6% Sb(III) recovery within 20 h.â¢SO42â can be simultaneously reduced to HSâ in Sb-acclimated anaerobic sludge.â¢The formed S2â participated in both Sb(V) reduction and Sb(III) precipitation.â¢Formation of Sb2O3 and Sb2S3 precipitates were confirmed.â¢SO42â stimulated the growth of Pseudomonas and sulfate-reducing bacteria.
The priority pollutant antimony (Sb) exists primarily as Sb(V) and Sb(III) in natural waters, and Sb(III) is generally with greater mobility and toxicity than Sb(V). The bio-reduction of Sb(V) would not become a meaningful Sb-removal process unless the accumulation of produced dissolved Sb(III) could be controlled. Here, we examined the dissimilatory antimonate bio-reduction with or without the coexistence of sulfate using Sb-acclimated biomass. Results demonstrated that 0.8 mM Sb(V) was almost completely bio-reduced within 20 h along with 48.6% Sb(III) recovery. Kinetic parameters qmax and Ks calculated were 0.54 mg-Sb mg-DWâ1 hâ1 and 41.96 mg Lâ1, respectively. When the concentrations of coexisting sulfate were 0.8 mM, 1.6 mM, and 4 mM, the reduction of 0.8 mM Sb(V) was accomplished within 17, 9, and 5 h, respectively, along with no final Sb(III) recovery. Also, the bio-reduction of sulfate occurred synchronously. The precipitated Sb2O3 and Sb2S3 were characterized by scanning electron microscopy coupled with energy dispersive spectrometer, X-ray diffraction, and X-ray photoelectron spectroscopy. Compared with bacterial compositions of the seed sludge obtained from anaerobic digestion tank in a wastewater treatment plant, new genera of Pseudomonas and Geobacter emerged with large proportions in both Sb-fed and Sb-sulfate-fed sludge, and a small portion of sulfate reduction bacteria emerged only in Sb-sulfate-fed culture.
Graphical abstractDownload high-res image (232KB)Download full-size image