| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4979254 | Journal of Hazardous Materials | 2018 | 8 Pages |
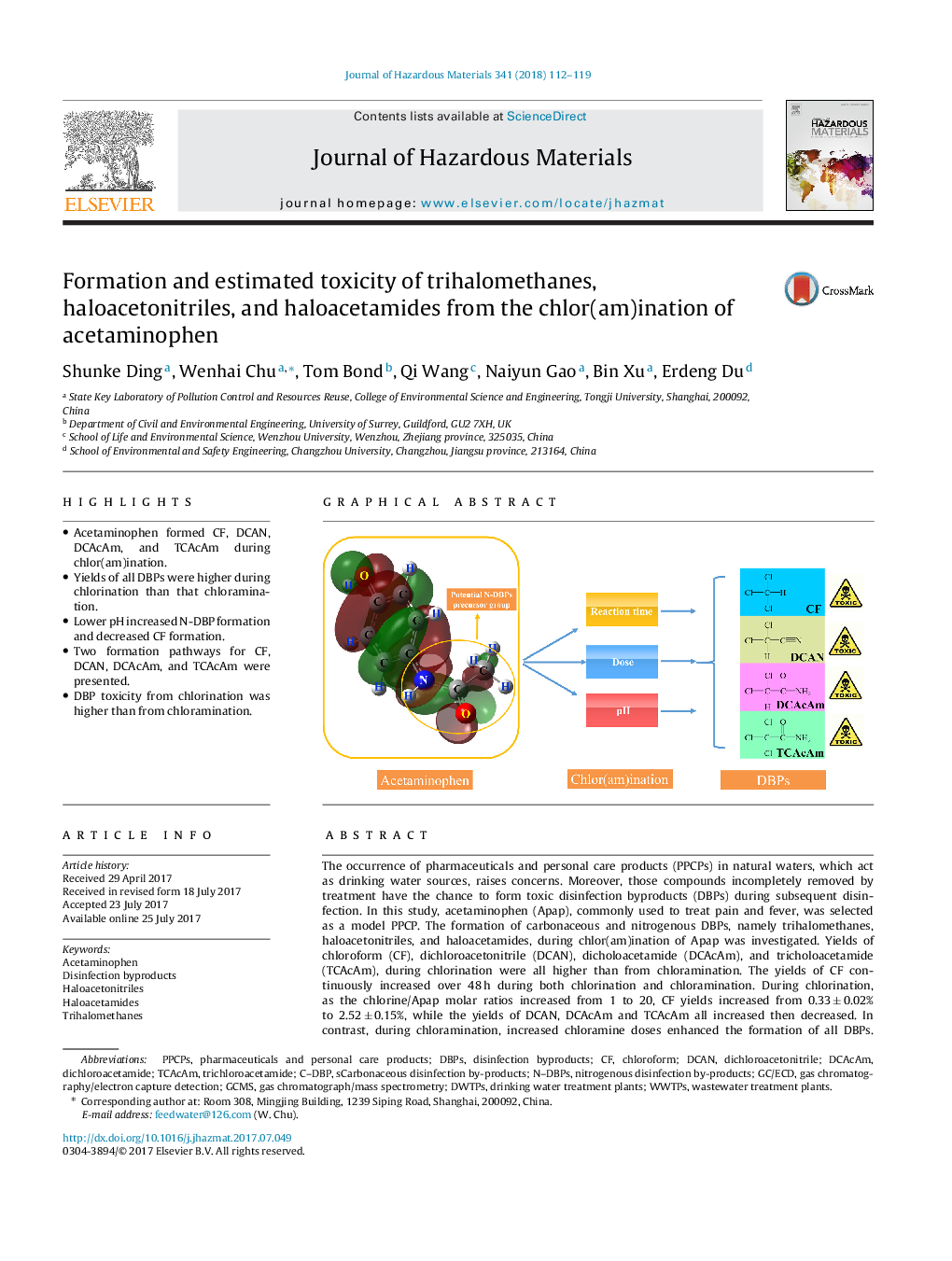
â¢Acetaminophen formed CF, DCAN, DCAcAm, and TCAcAm during chlor(am)ination.â¢Yields of all DBPs were higher during chlorination than that chloramination.â¢Lower pH increased N-DBP formation and decreased CF formation.â¢Two formation pathways for CF, DCAN, DCAcAm, and TCAcAm were presented.â¢DBP toxicity from chlorination was higher than from chloramination.
The occurrence of pharmaceuticals and personal care products (PPCPs) in natural waters, which act as drinking water sources, raises concerns. Moreover, those compounds incompletely removed by treatment have the chance to form toxic disinfection byproducts (DBPs) during subsequent disinfection. In this study, acetaminophen (Apap), commonly used to treat pain and fever, was selected as a model PPCP. The formation of carbonaceous and nitrogenous DBPs, namely trihalomethanes, haloacetonitriles, and haloacetamides, during chlor(am)ination of Apap was investigated. Yields of chloroform (CF), dichloroacetonitrile (DCAN), dicholoacetamide (DCAcAm), and tricholoacetamide (TCAcAm), during chlorination were all higher than from chloramination. The yields of CF continuously increased over 48 h during both chlorination and chloramination. During chlorination, as the chlorine/Apap molar ratios increased from 1 to 20, CF yields increased from 0.33 ± 0.02% to 2.52 ± 0.15%, while the yields of DCAN, DCAcAm and TCAcAm all increased then decreased. In contrast, during chloramination, increased chloramine doses enhanced the formation of all DBPs. Acidic conditions favored nitrogenous DBP formation, regardless of chlorination or chloramination, whereas alkaline conditions enhanced CF formation. Two proposed formation mechanisms are presented. The analysed DBPs formed during chlorination were 2 orders of magnitude more genotoxic and cytotoxicity than those from chloramination.
Graphical abstractDownload high-res image (198KB)Download full-size image