| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 4979263 | Journal of Hazardous Materials | 2018 | 9 Pages |
â¢Magnetic core-shell Fe3O4@mSiO2-NH2 adsorbent was prepared and its tunable removal efficiency for Fe3+ was studied.â¢The adsorption process follows the pseudo-second-order model and the Langmuir model.â¢A two-stage adsorption process of Fe3O4@mSiO2-NH2 for Fe3+ was developed to explore the adsorption mechanism.â¢Fe3O4@mSiO2-NH2 could be effectively regenerated after adsorption and the removal efficiency has little change.
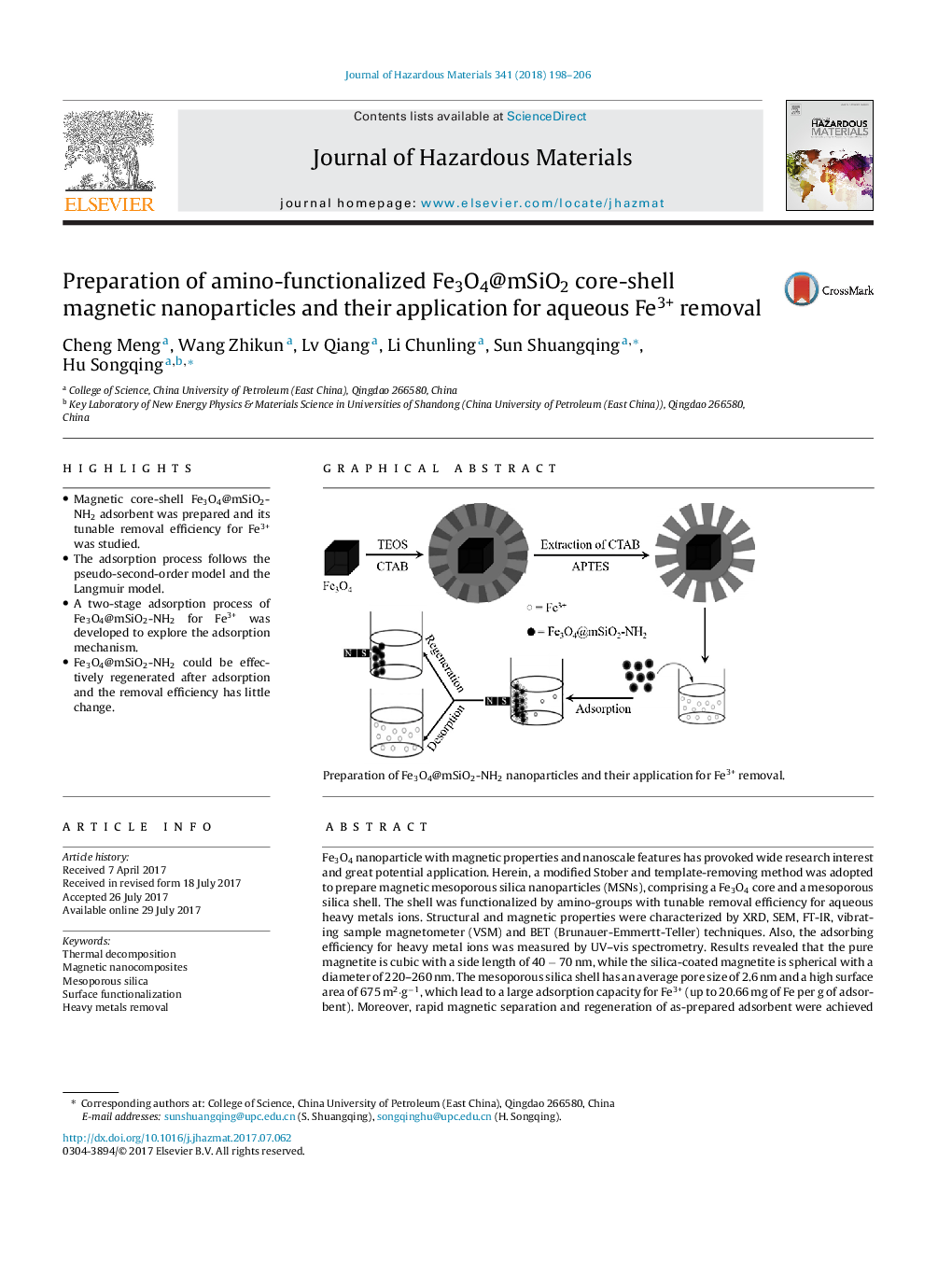
Fe3O4 nanoparticle with magnetic properties and nanoscale features has provoked wide research interest and great potential application. Herein, a modified Stober and template-removing method was adopted to prepare magnetic mesoporous silica nanoparticles (MSNs), comprising a Fe3O4 core and a mesoporous silica shell. The shell was functionalized by amino-groups with tunable removal efficiency for aqueous heavy metals ions. Structural and magnetic properties were characterized by XRD, SEM, FT-IR, vibrating sample magnetometer (VSM) and BET (Brunauer-Emmertt-Teller) techniques. Also, the adsorbing efficiency for heavy metal ions was measured by UV-vis spectrometry. Results revealed that the pure magnetite is cubic with a side length of 40 â 70 nm, while the silica-coated magnetite is spherical with a diameter of 220-260 nm. The mesoporous silica shell has an average pore size of 2.6 nm and a high surface area of 675 m2·gâ1, which lead to a large adsorption capacity for Fe3+ (up to 20.66 mg of Fe per g of adsorbent). Moreover, rapid magnetic separation and regeneration of as-prepared adsorbent were achieved conveniently. The distinctive structure and the heavy metal ions removal property of magnetic nanocomposites reflect their prospective application in water treatment.
Graphical abstractDownload high-res image (116KB)Download full-size imagePreparation of Fe3O4@mSiO2-NH2 nanoparticles and their application for Fe3+ removal.