| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5187892 | Polymer | 2008 | 8 Pages |
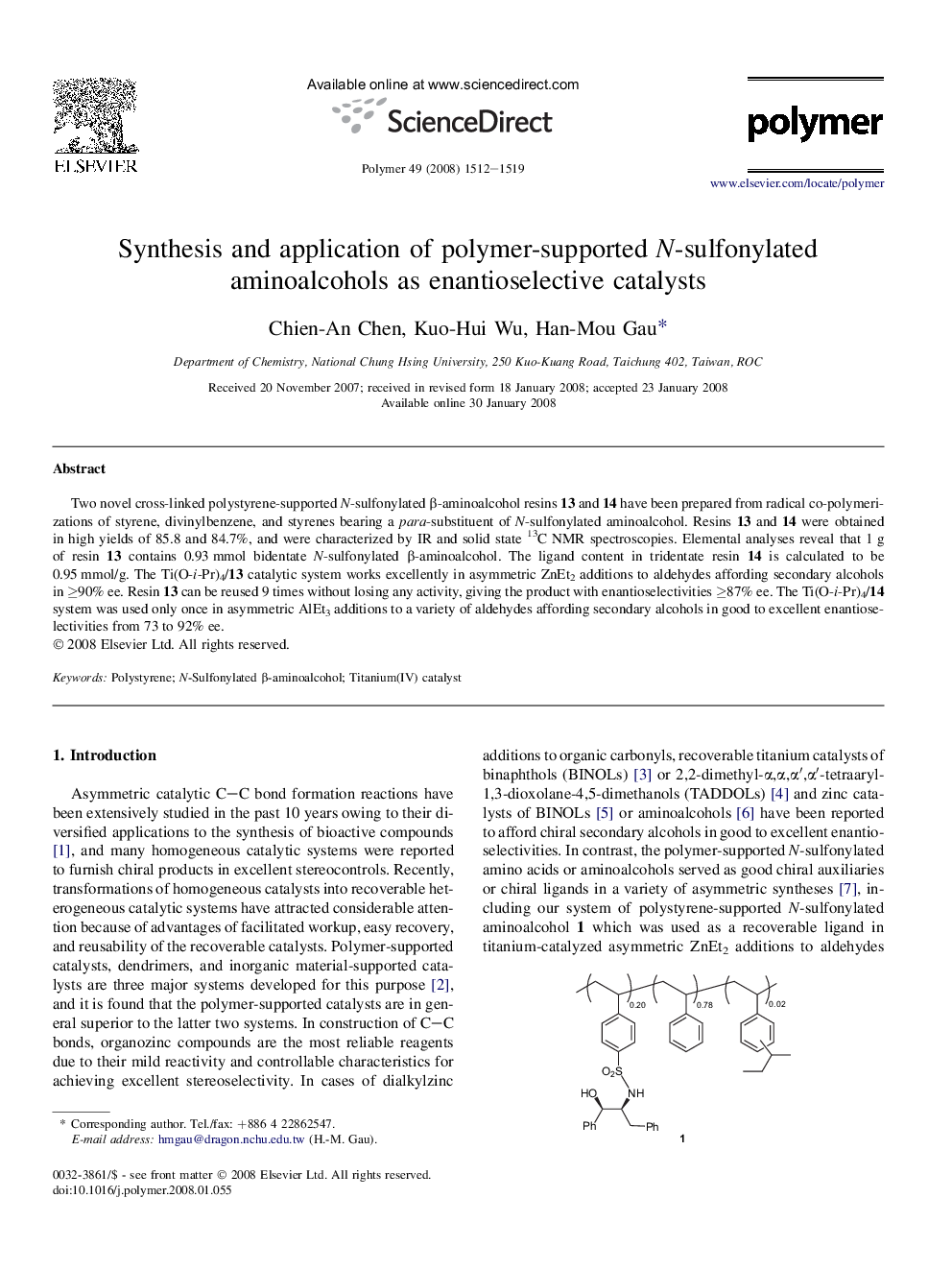
Two novel cross-linked polystyrene-supported N-sulfonylated β-aminoalcohol resins 13 and 14 have been prepared from radical co-polymerizations of styrene, divinylbenzene, and styrenes bearing a para-substituent of N-sulfonylated aminoalcohol. Resins 13 and 14 were obtained in high yields of 85.8 and 84.7%, and were characterized by IR and solid state 13C NMR spectroscopies. Elemental analyses reveal that 1 g of resin 13 contains 0.93 mmol bidentate N-sulfonylated β-aminoalcohol. The ligand content in tridentate resin 14 is calculated to be 0.95 mmol/g. The Ti(O-i-Pr)4/13 catalytic system works excellently in asymmetric ZnEt2 additions to aldehydes affording secondary alcohols in â¥90% ee. Resin 13 can be reused 9 times without losing any activity, giving the product with enantioselectivities â¥87% ee. The Ti(O-i-Pr)4/14 system was used only once in asymmetric AlEt3 additions to a variety of aldehydes affording secondary alcohols in good to excellent enantioselectivities from 73 to 92% ee.