| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5212294 | Tetrahedron | 2017 | 8 Pages |
Abstract
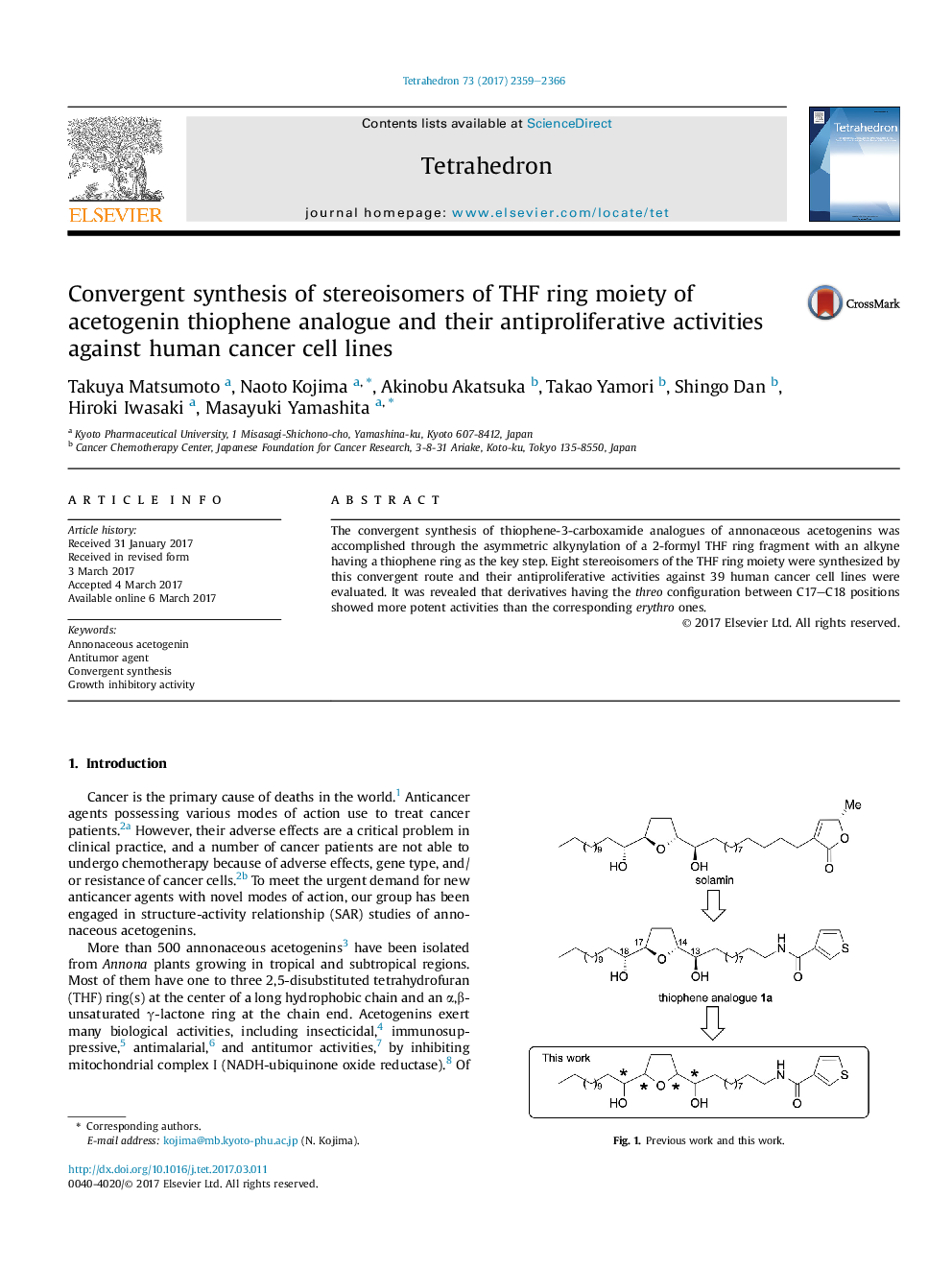
The convergent synthesis of thiophene-3-carboxamide analogues of annonaceous acetogenins was accomplished through the asymmetric alkynylation of a 2-formyl THF ring fragment with an alkyne having a thiophene ring as the key step. Eight stereoisomers of the THF ring moiety were synthesized by this convergent route and their antiproliferative activities against 39 human cancer cell lines were evaluated. It was revealed that derivatives having the threo configuration between C17-C18 positions showed more potent activities than the corresponding erythro ones.
Graphical abstractDownload high-res image (187KB)Download full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Takuya Matsumoto, Naoto Kojima, Akinobu Akatsuka, Takao Yamori, Shingo Dan, Hiroki Iwasaki, Masayuki Yamashita,