| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5213134 | Tetrahedron | 2016 | 5 Pages |
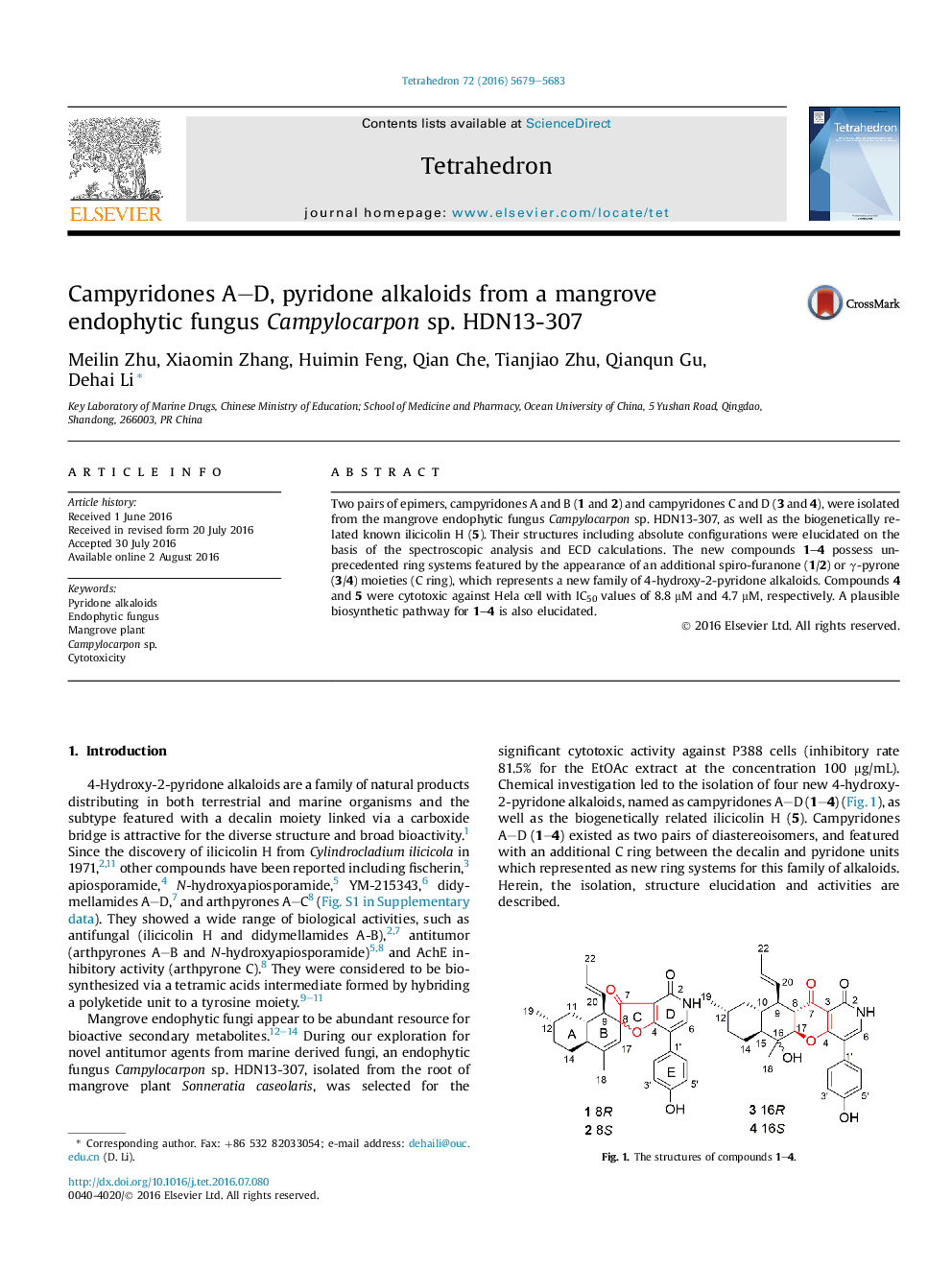
Two pairs of epimers, campyridones A and B (1 and 2) and campyridones C and D (3 and 4), were isolated from the mangrove endophytic fungus Campylocarpon sp. HDN13-307, as well as the biogenetically related known ilicicolin H (5). Their structures including absolute configurations were elucidated on the basis of the spectroscopic analysis and ECD calculations. The new compounds 1-4 possess unprecedented ring systems featured by the appearance of an additional spiro-furanone (1/2) or γ-pyrone (3/4) moieties (C ring), which represents a new family of 4-hydroxy-2-pyridone alkaloids. Compounds 4 and 5 were cytotoxic against Hela cell with IC50 values of 8.8 μM and 4.7 μM, respectively. A plausible biosynthetic pathway for 1-4 is also elucidated.
Graphical abstractDownload full-size image