| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5215485 | Tetrahedron | 2015 | 9 Pages |
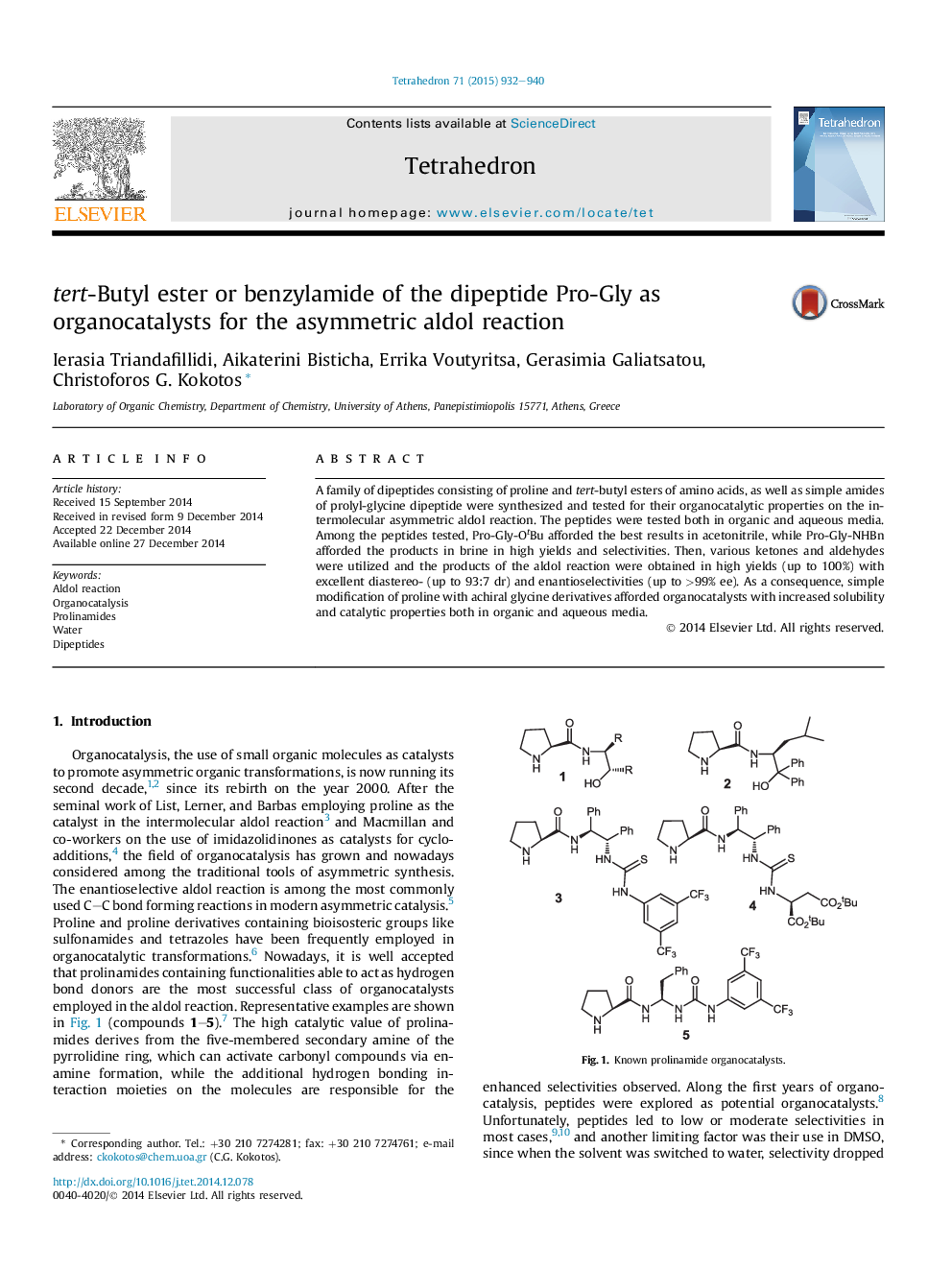
A family of dipeptides consisting of proline and tert-butyl esters of amino acids, as well as simple amides of prolyl-glycine dipeptide were synthesized and tested for their organocatalytic properties on the intermolecular asymmetric aldol reaction. The peptides were tested both in organic and aqueous media. Among the peptides tested, Pro-Gly-OtBu afforded the best results in acetonitrile, while Pro-Gly-NHBn afforded the products in brine in high yields and selectivities. Then, various ketones and aldehydes were utilized and the products of the aldol reaction were obtained in high yields (up to 100%) with excellent diastereo- (up to 93:7 dr) and enantioselectivities (up to >99% ee). As a consequence, simple modification of proline with achiral glycine derivatives afforded organocatalysts with increased solubility and catalytic properties both in organic and aqueous media.
Graphical abstractDownload full-size image