| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5217993 | Tetrahedron | 2013 | 13 Pages |
Abstract
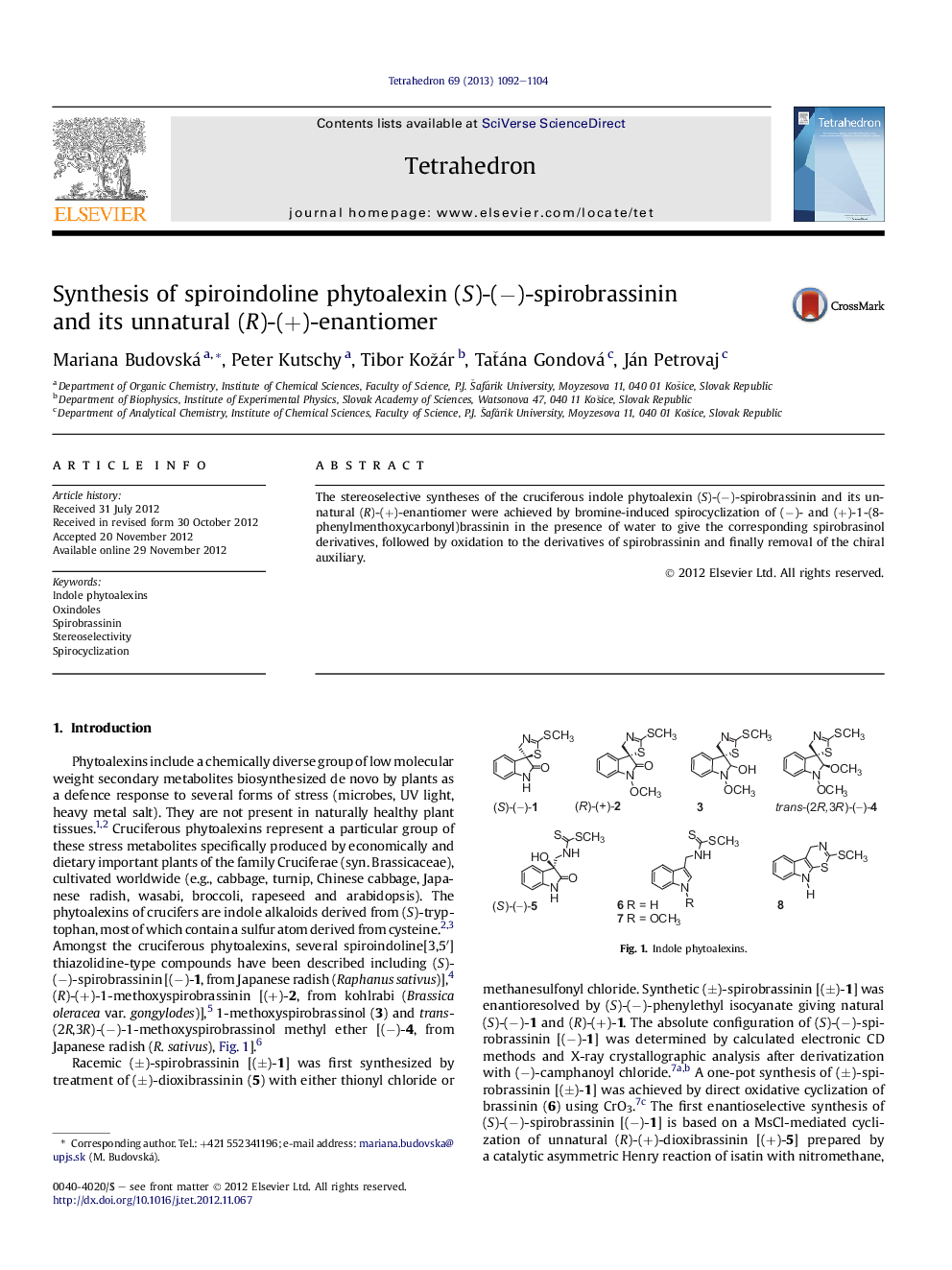
The stereoselective syntheses of the cruciferous indole phytoalexin (S)-(â)-spirobrassinin and its unnatural (R)-(+)-enantiomer were achieved by bromine-induced spirocyclization of (â)- and (+)-1-(8-phenylmenthoxycarbonyl)brassinin in the presence of water to give the corresponding spirobrasinol derivatives, followed by oxidation to the derivatives of spirobrassinin and finally removal of the chiral auxiliary.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Mariana Budovská, Peter Kutschy, Tibor Kožár, TaÅ¥ána Gondová, Ján Petrovaj,