| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5218139 | Tetrahedron | 2013 | 7 Pages |
Abstract
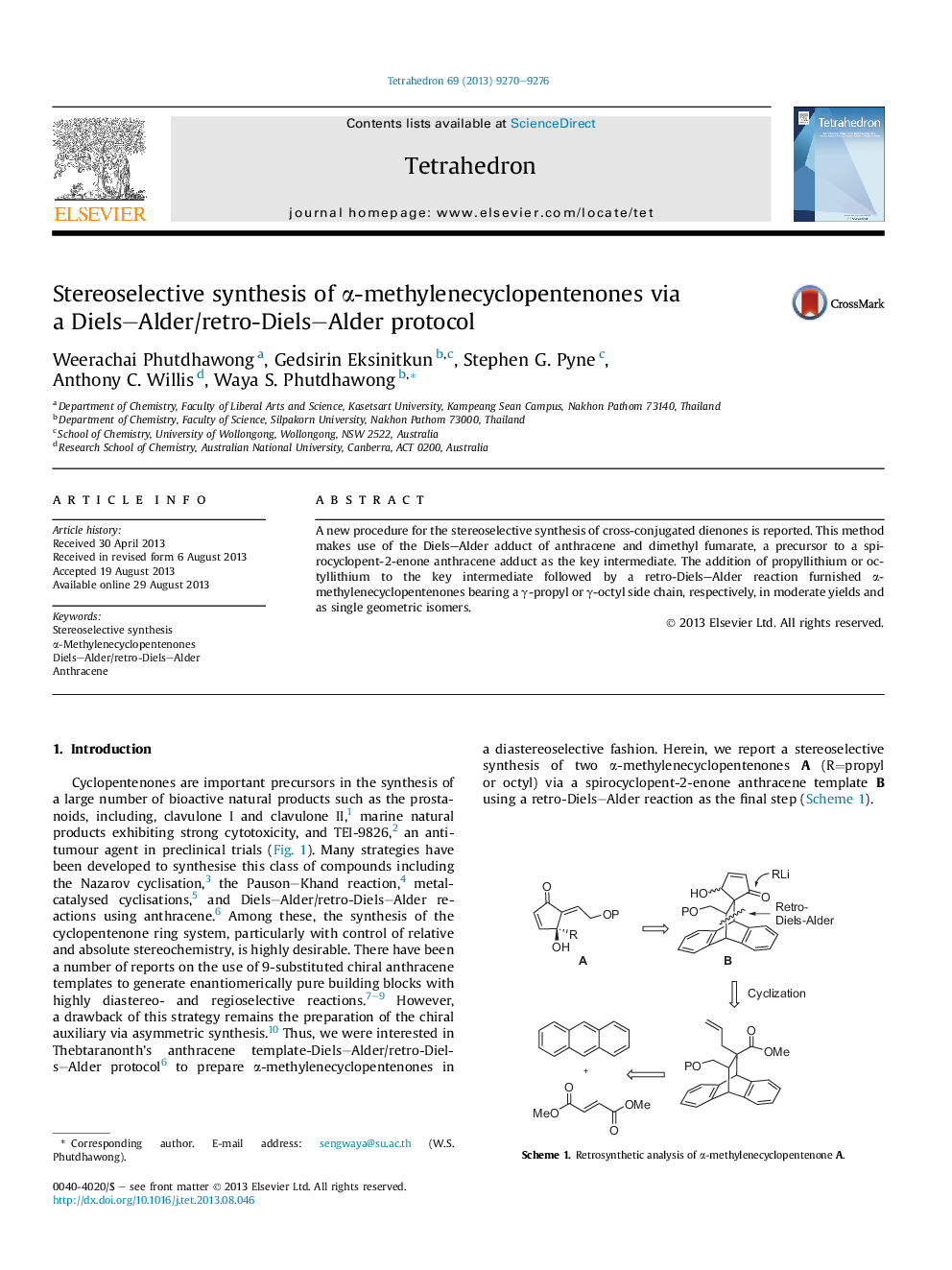
A new procedure for the stereoselective synthesis of cross-conjugated dienones is reported. This method makes use of the Diels–Alder adduct of anthracene and dimethyl fumarate, a precursor to a spirocyclopent-2-enone anthracene adduct as the key intermediate. The addition of propyllithium or octyllithium to the key intermediate followed by a retro-Diels–Alder reaction furnished α-methylenecyclopentenones bearing a γ-propyl or γ-octyl side chain, respectively, in moderate yields and as single geometric isomers.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry