| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5218260 | Tetrahedron | 2013 | 7 Pages |
Abstract
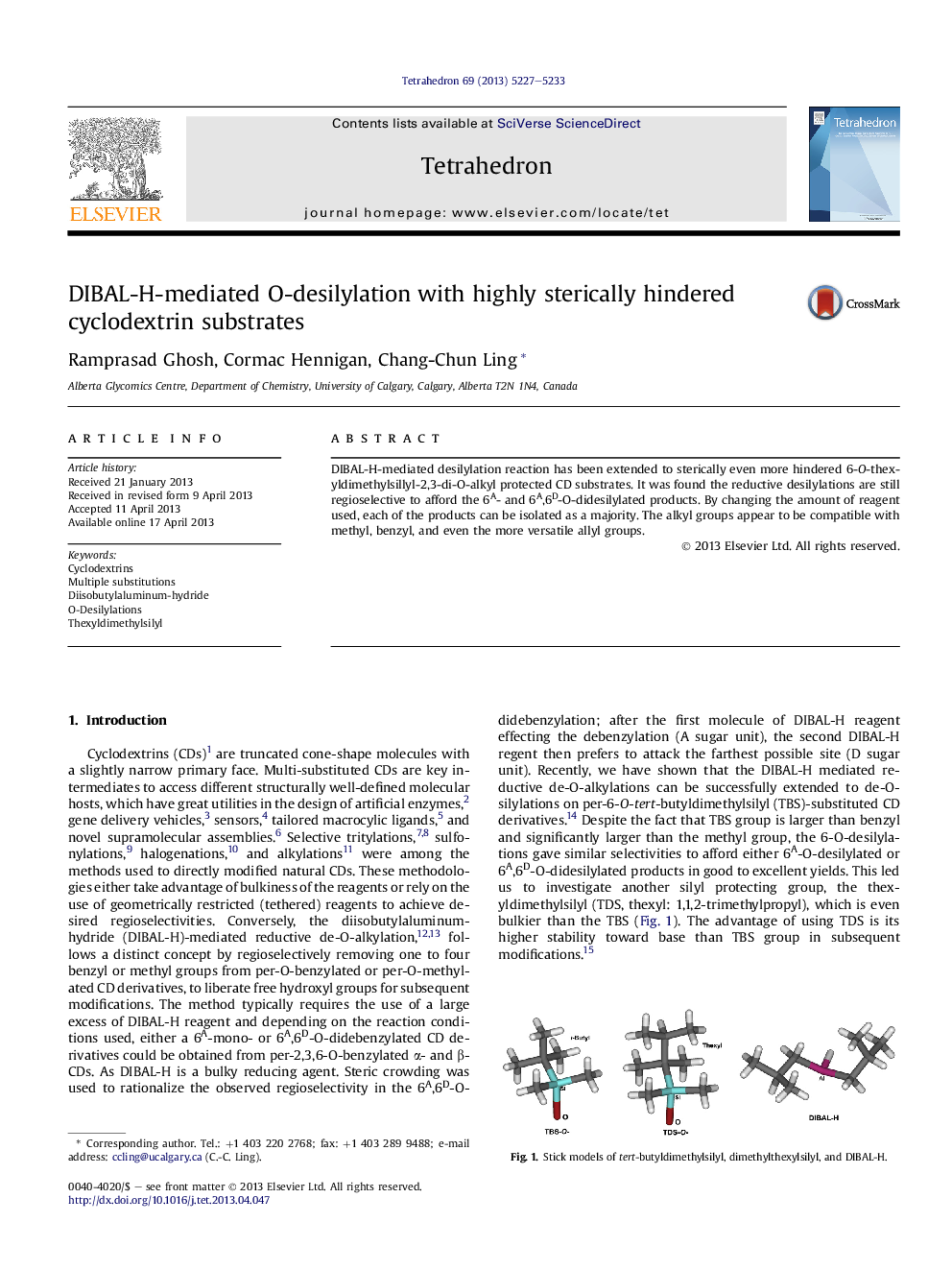
DIBAL-H-mediated desilylation reaction has been extended to sterically even more hindered 6-O-thexyldimethylsillyl-2,3-di-O-alkyl protected CD substrates. It was found the reductive desilylations are still regioselective to afford the 6A- and 6A,6D-O-didesilylated products. By changing the amount of reagent used, each of the products can be isolated as a majority. The alkyl groups appear to be compatible with methyl, benzyl, and even the more versatile allyl groups.
Graphical abstractDownload full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Ramprasad Ghosh, Cormac Hennigan, Chang-Chun Ling,