| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5219091 | Tetrahedron | 2012 | 8 Pages |
Abstract
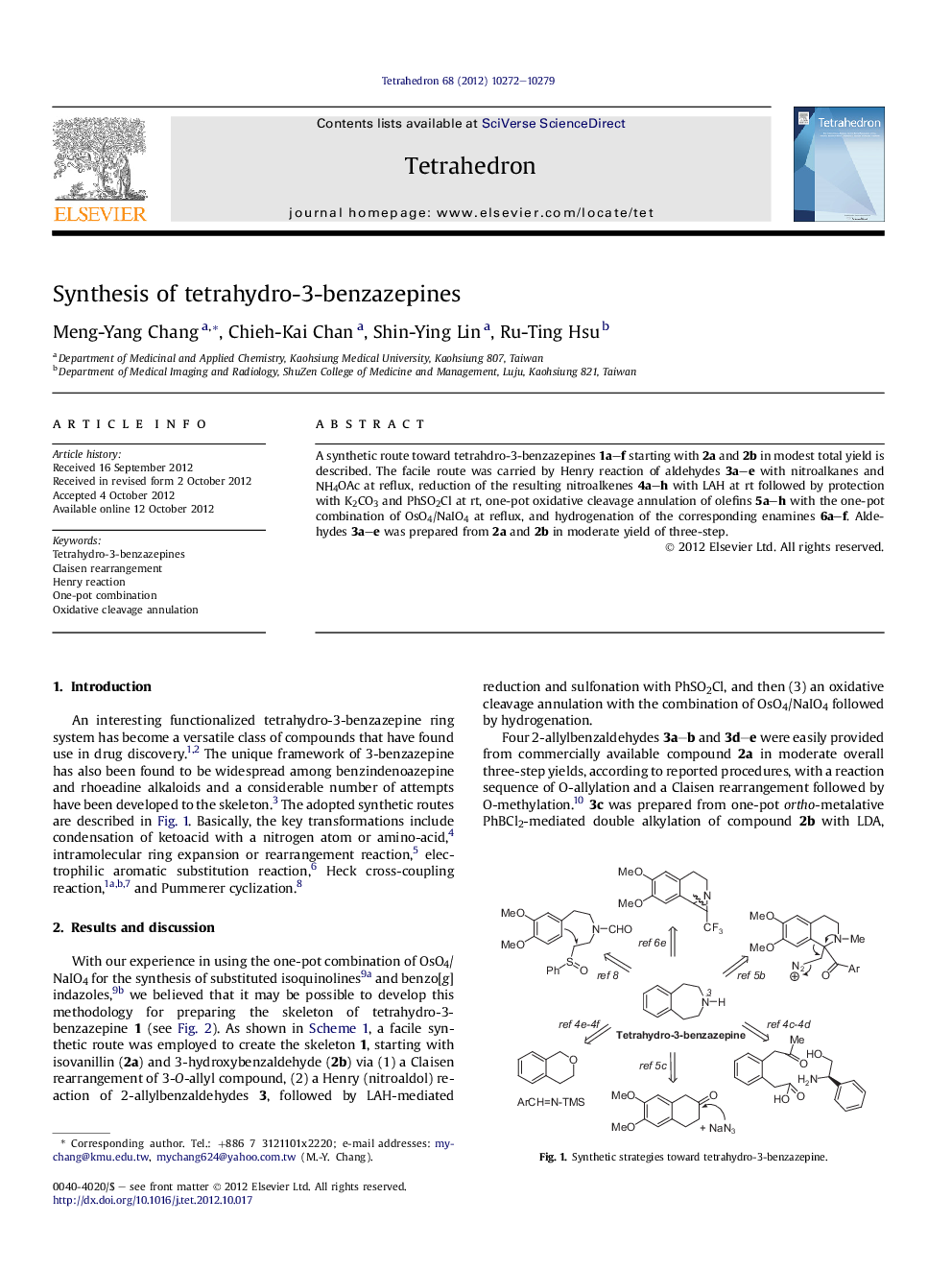
A synthetic route toward tetrahdro-3-benzazepines 1a-f starting with 2a and 2b in modest total yield is described. The facile route was carried by Henry reaction of aldehydes 3a-e with nitroalkanes and NH4OAc at reflux, reduction of the resulting nitroalkenes 4a-h with LAH at rt followed by protection with K2CO3 and PhSO2Cl at rt, one-pot oxidative cleavage annulation of olefins 5a-h with the one-pot combination of OsO4/NaIO4 at reflux, and hydrogenation of the corresponding enamines 6a-f. Aldehydes 3a-e was prepared from 2a and 2b in moderate yield of three-step.
Graphical abstractDownload full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Meng-Yang Chang, Chieh-Kai Chan, Shin-Ying Lin, Ru-Ting Hsu,