| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5219228 | Tetrahedron | 2012 | 10 Pages |
Abstract
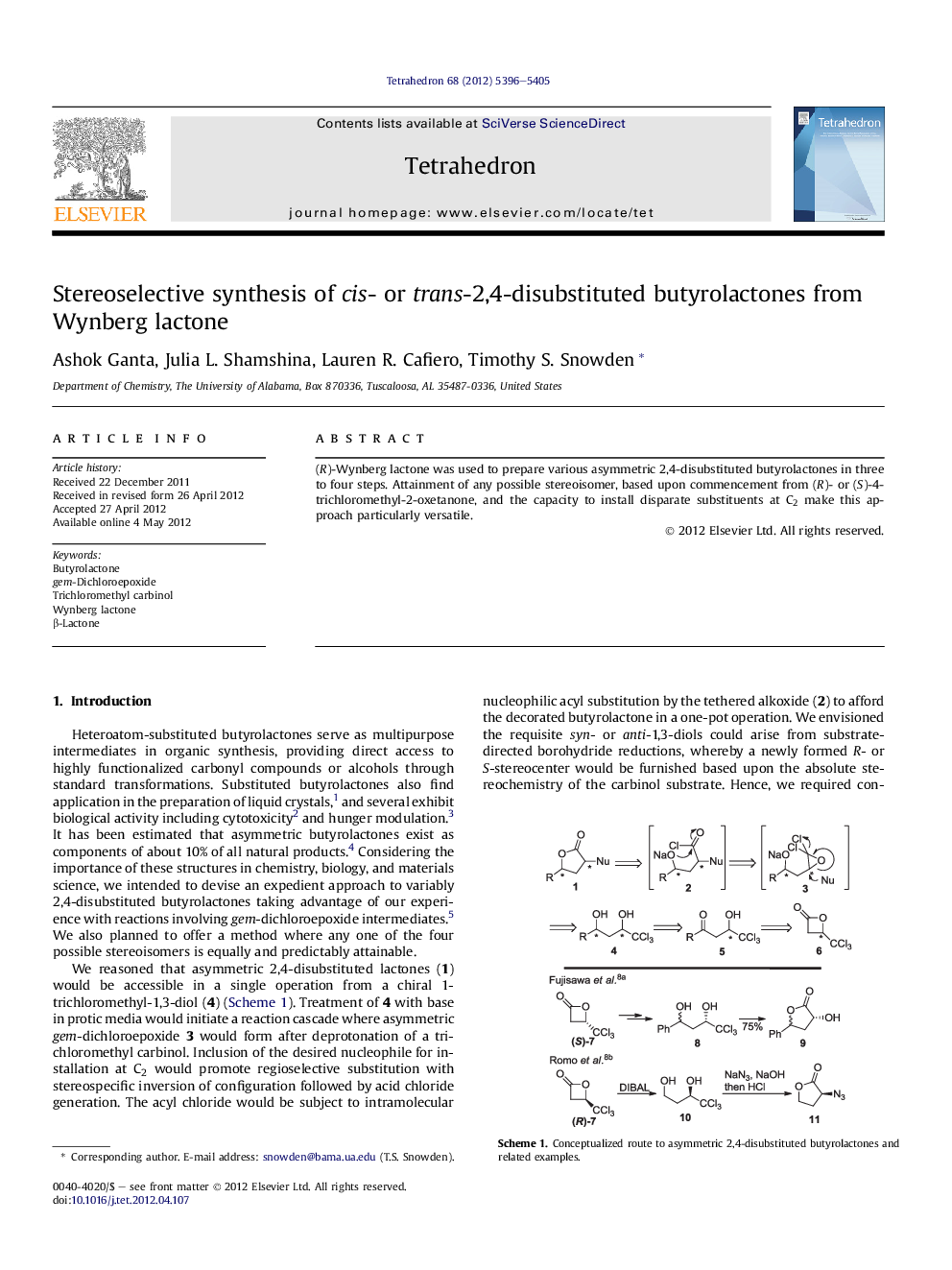
(R)-Wynberg lactone was used to prepare various asymmetric 2,4-disubstituted butyrolactones in three to four steps. Attainment of any possible stereoisomer, based upon commencement from (R)- or (S)-4-trichloromethyl-2-oxetanone, and the capacity to install disparate substituents at C2 make this approach particularly versatile.
Graphical abstractDownload full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Ashok Ganta, Julia L. Shamshina, Lauren R. Cafiero, Timothy S. Snowden,