| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5219337 | Tetrahedron | 2011 | 9 Pages |
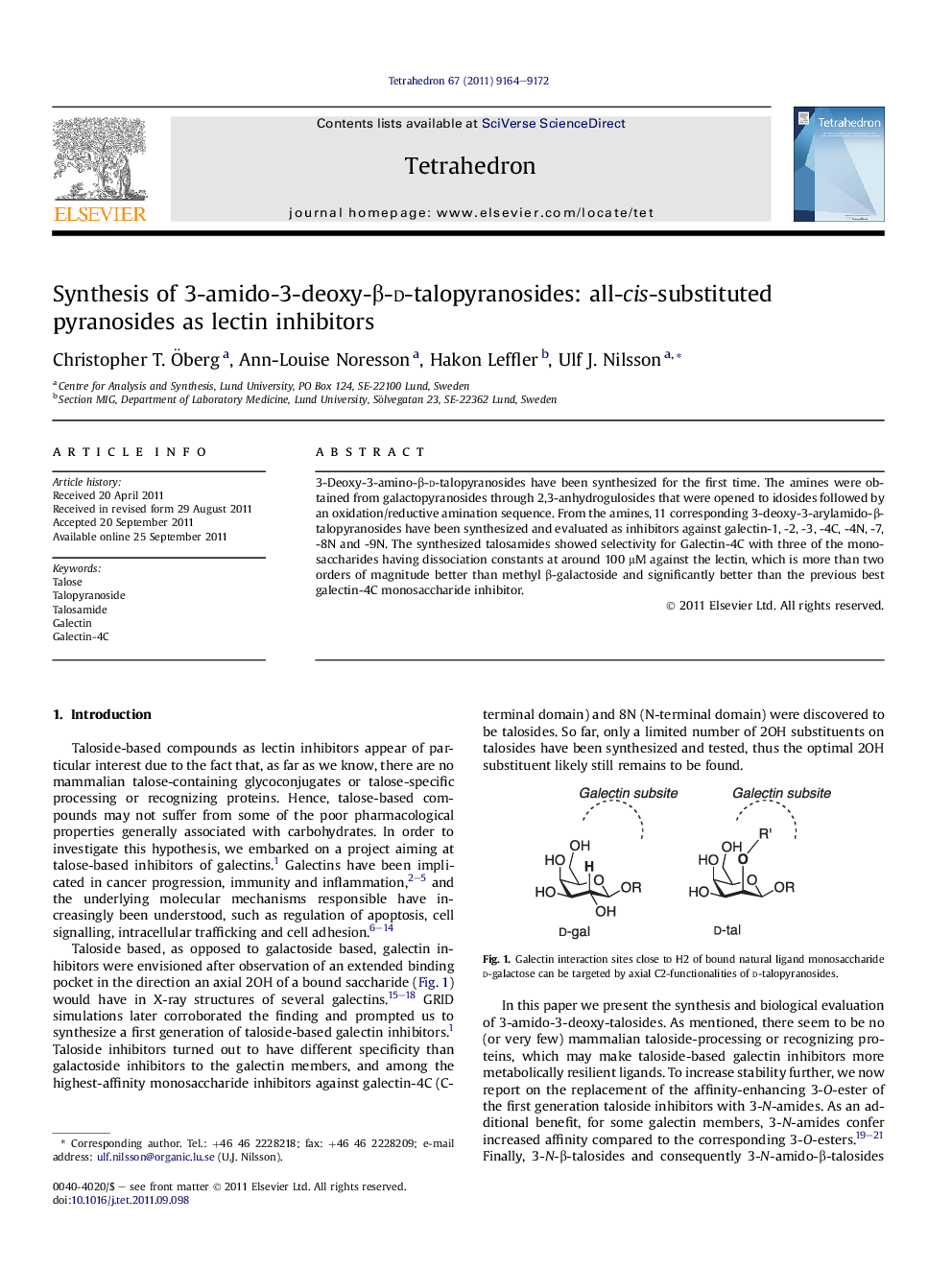
3-Deoxy-3-amino-β-d-talopyranosides have been synthesized for the first time. The amines were obtained from galactopyranosides through 2,3-anhydrogulosides that were opened to idosides followed by an oxidation/reductive amination sequence. From the amines, 11 corresponding 3-deoxy-3-arylamido-β-talopyranosides have been synthesized and evaluated as inhibitors against galectin-1, -2, -3, -4C, -4N, -7, -8N and -9N. The synthesized talosamides showed selectivity for Galectin-4C with three of the monosaccharides having dissociation constants at around 100 μM against the lectin, which is more than two orders of magnitude better than methyl β-galactoside and significantly better than the previous best galectin-4C monosaccharide inhibitor.
Graphical abstractDownload full-size image