| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5219724 | Tetrahedron | 2012 | 6 Pages |
Abstract
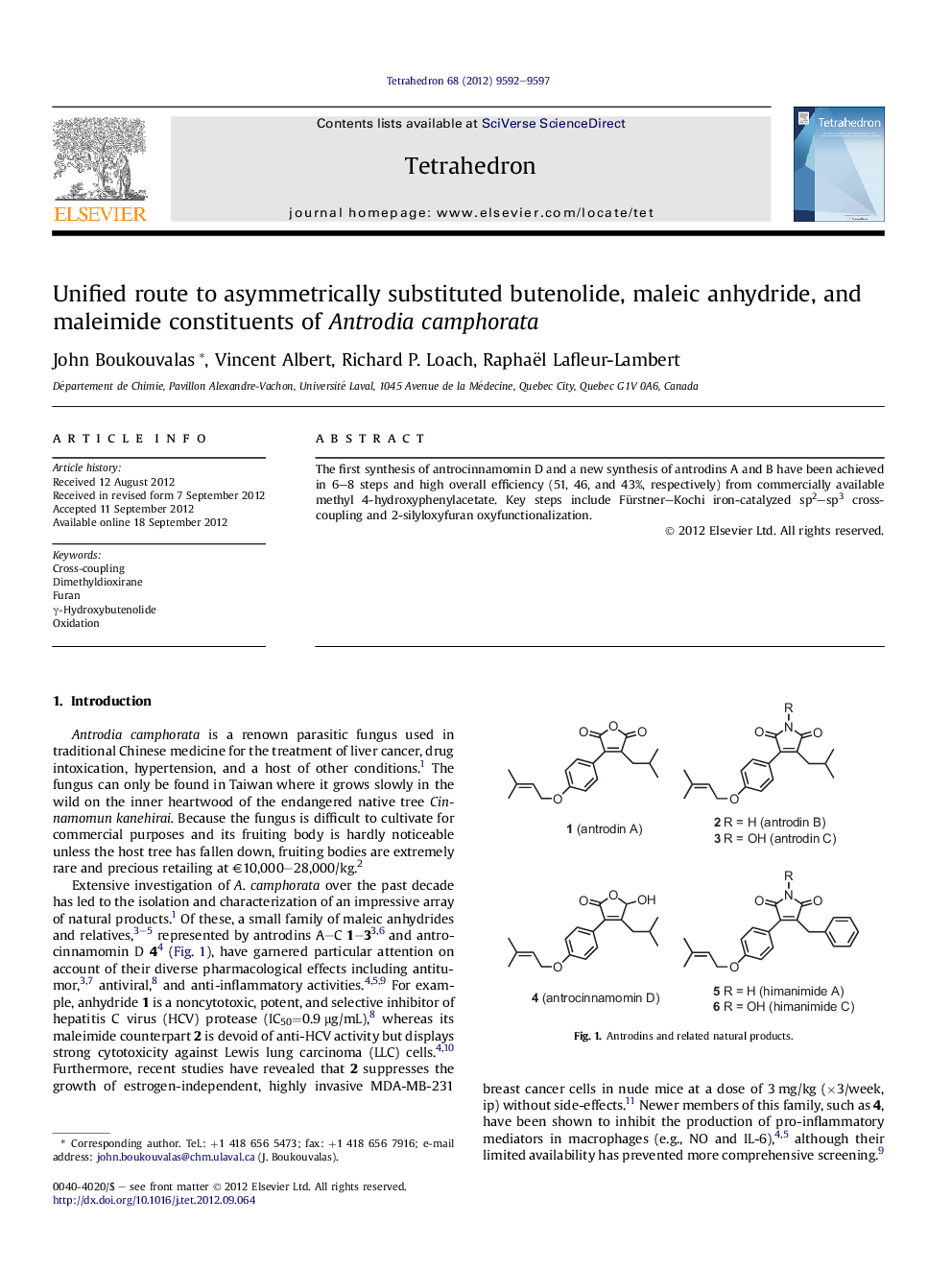
The first synthesis of antrocinnamomin D and a new synthesis of antrodins A and B have been achieved in 6-8 steps and high overall efficiency (51, 46, and 43%, respectively) from commercially available methyl 4-hydroxyphenylacetate. Key steps include Fürstner-Kochi iron-catalyzed sp2-sp3 cross-coupling and 2-silyloxyfuran oxyfunctionalization.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
John Boukouvalas, Vincent Albert, Richard P. Loach, Raphaël Lafleur-Lambert,