| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5220146 | Tetrahedron | 2012 | 6 Pages |
Abstract
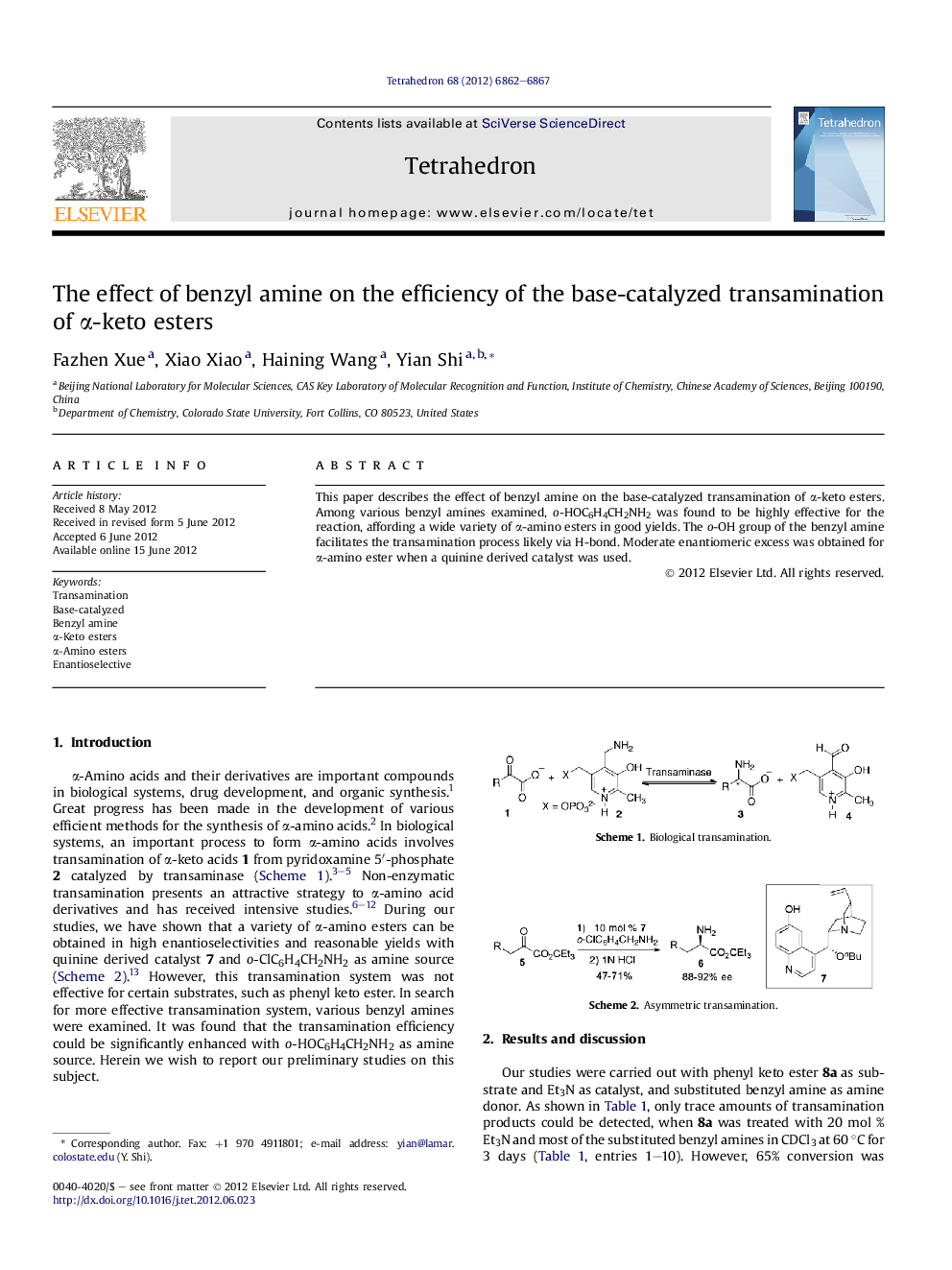
This paper describes the effect of benzyl amine on the base-catalyzed transamination of α-keto esters. Among various benzyl amines examined, o-HOC6H4CH2NH2 was found to be highly effective for the reaction, affording a wide variety of α-amino esters in good yields. The o-OH group of the benzyl amine facilitates the transamination process likely via H-bond. Moderate enantiomeric excess was obtained for α-amino ester when a quinine derived catalyst was used.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Fazhen Xue, Xiao Xiao, Haining Wang, Yian Shi,