| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5220595 | Tetrahedron | 2011 | 6 Pages |
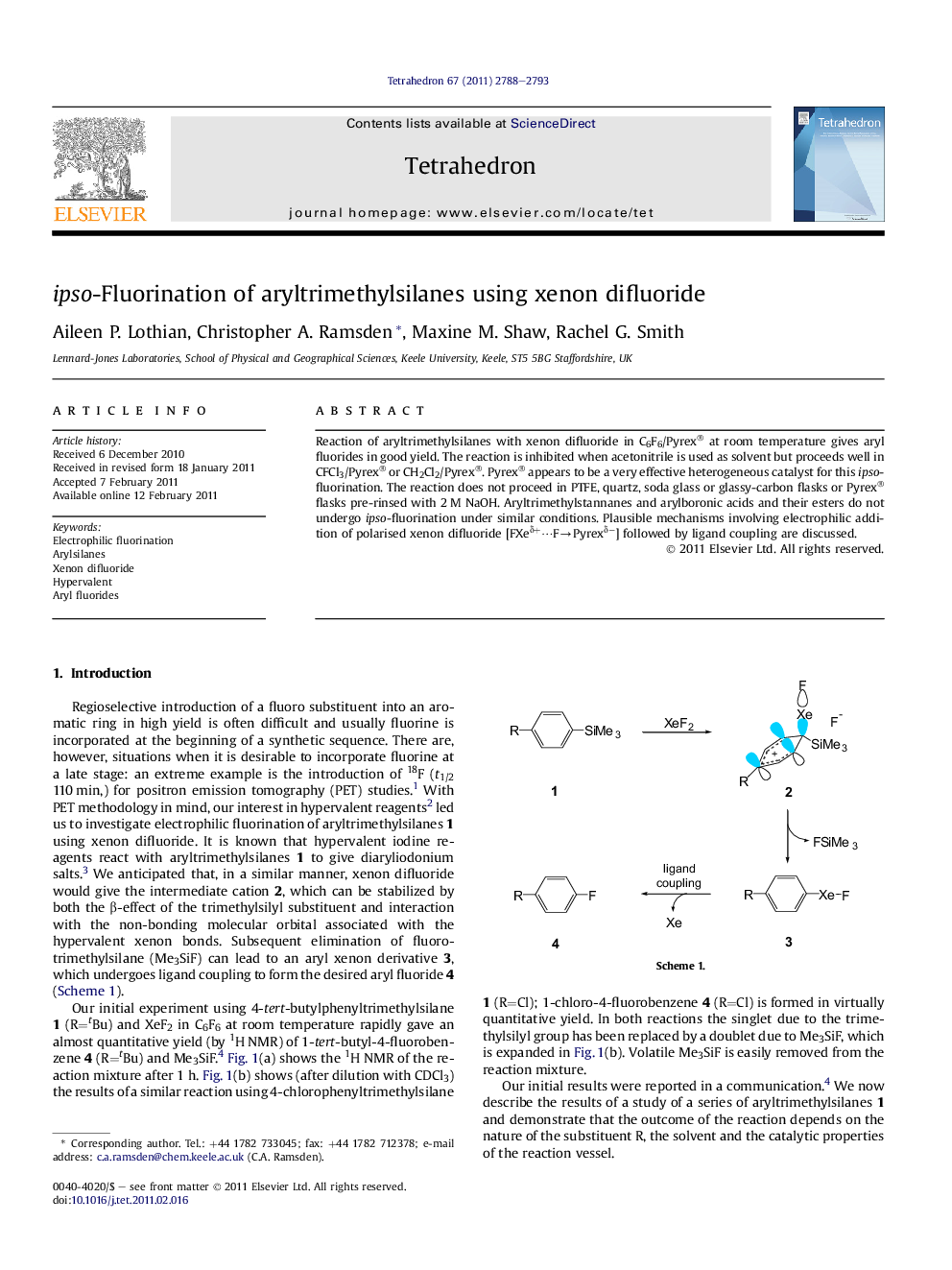
Reaction of aryltrimethylsilanes with xenon difluoride in C6F6/Pyrex® at room temperature gives aryl fluorides in good yield. The reaction is inhibited when acetonitrile is used as solvent but proceeds well in CFCl3/Pyrex® or CH2Cl2/Pyrex®. Pyrex® appears to be a very effective heterogeneous catalyst for this ipso-fluorination. The reaction does not proceed in PTFE, quartz, soda glass or glassy-carbon flasks or Pyrex® flasks pre-rinsed with 2 M NaOH. Aryltrimethylstannanes and arylboronic acids and their esters do not undergo ipso-fluorination under similar conditions. Plausible mechanisms involving electrophilic addition of polarised xenon difluoride [FXeδ+â¯FâPyrexδâ] followed by ligand coupling are discussed.
Graphical abstractDownload full-size image