| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5220829 | Tetrahedron | 2012 | 7 Pages |
Abstract
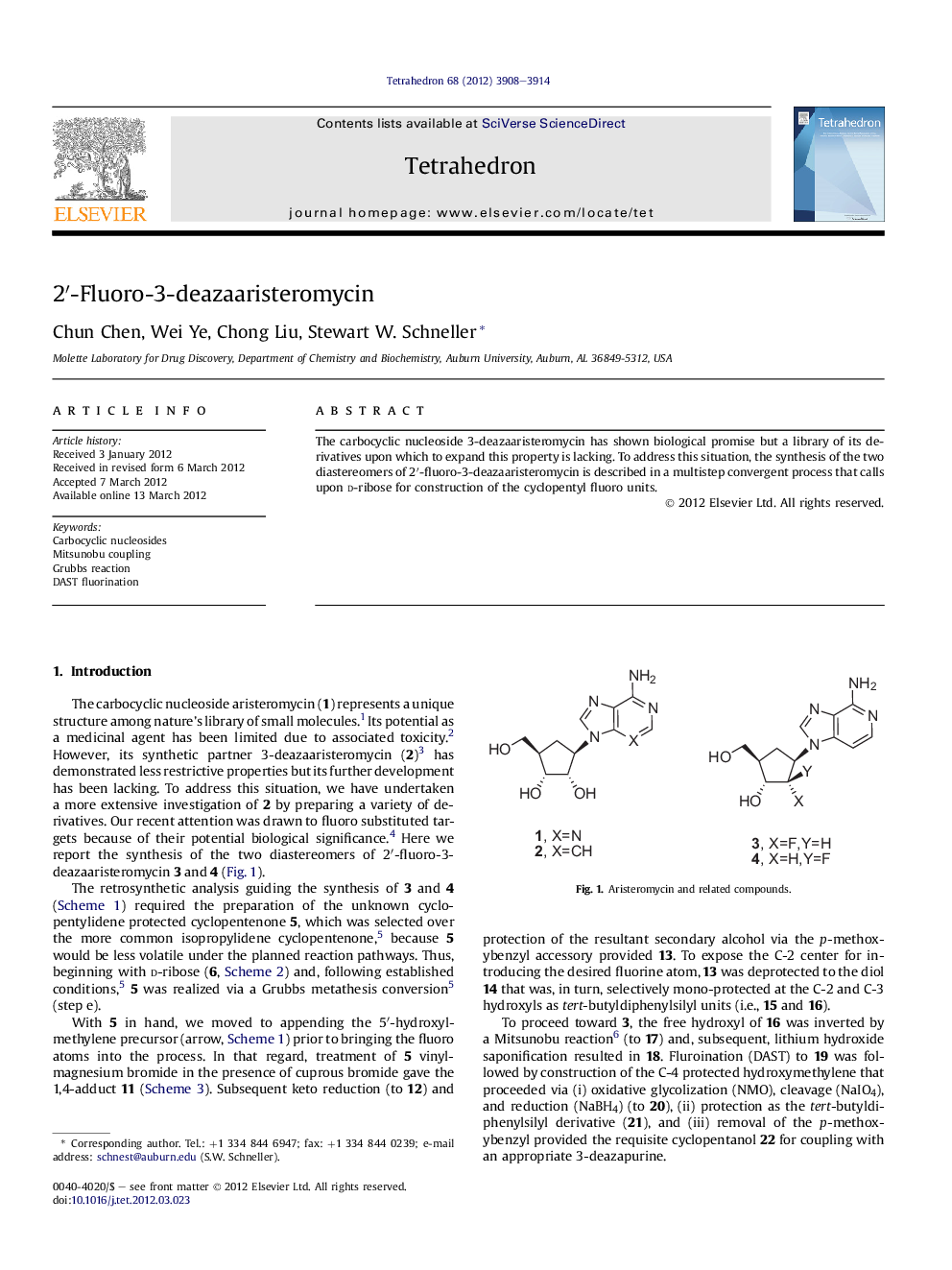
The carbocyclic nucleoside 3-deazaaristeromycin has shown biological promise but a library of its derivatives upon which to expand this property is lacking. To address this situation, the synthesis of the two diastereomers of 2â²-fluoro-3-deazaaristeromycin is described in a multistep convergent process that calls upon d-ribose for construction of the cyclopentyl fluoro units.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Chun Chen, Wei Ye, Chong Liu, Stewart W. Schneller,