| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5221048 | Tetrahedron | 2012 | 7 Pages |
Abstract
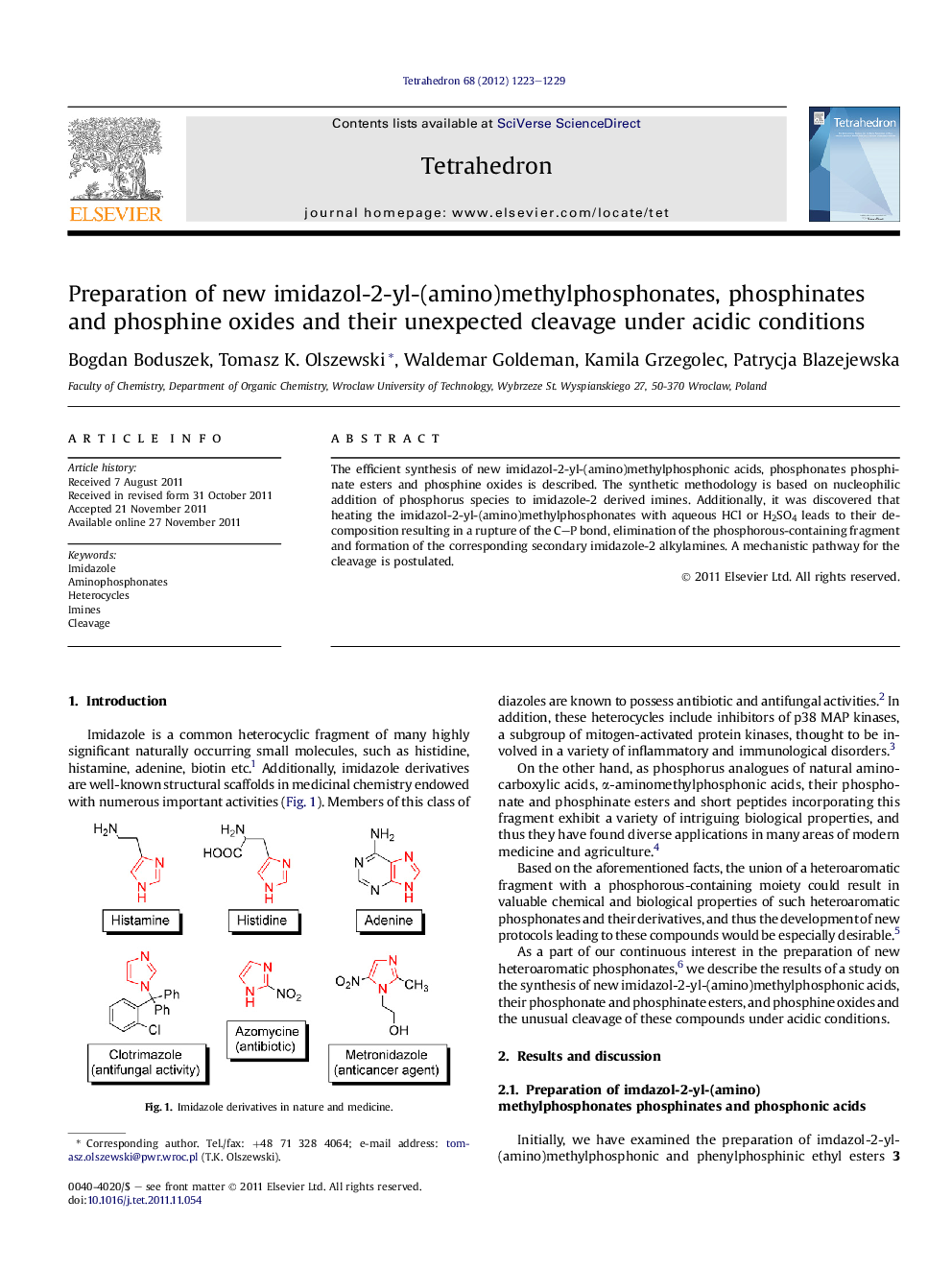
The efficient synthesis of new imidazol-2-yl-(amino)methylphosphonic acids, phosphonates phosphinate esters and phosphine oxides is described. The synthetic methodology is based on nucleophilic addition of phosphorus species to imidazole-2 derived imines. Additionally, it was discovered that heating the imidazol-2-yl-(amino)methylphosphonates with aqueous HCl or H2SO4 leads to their decomposition resulting in a rupture of the C-P bond, elimination of the phosphorous-containing fragment and formation of the corresponding secondary imidazole-2 alkylamines. A mechanistic pathway for the cleavage is postulated.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Bogdan Boduszek, Tomasz K. Olszewski, Waldemar Goldeman, Kamila Grzegolec, Patrycja Blazejewska,