| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5221121 | Tetrahedron | 2011 | 5 Pages |
Abstract
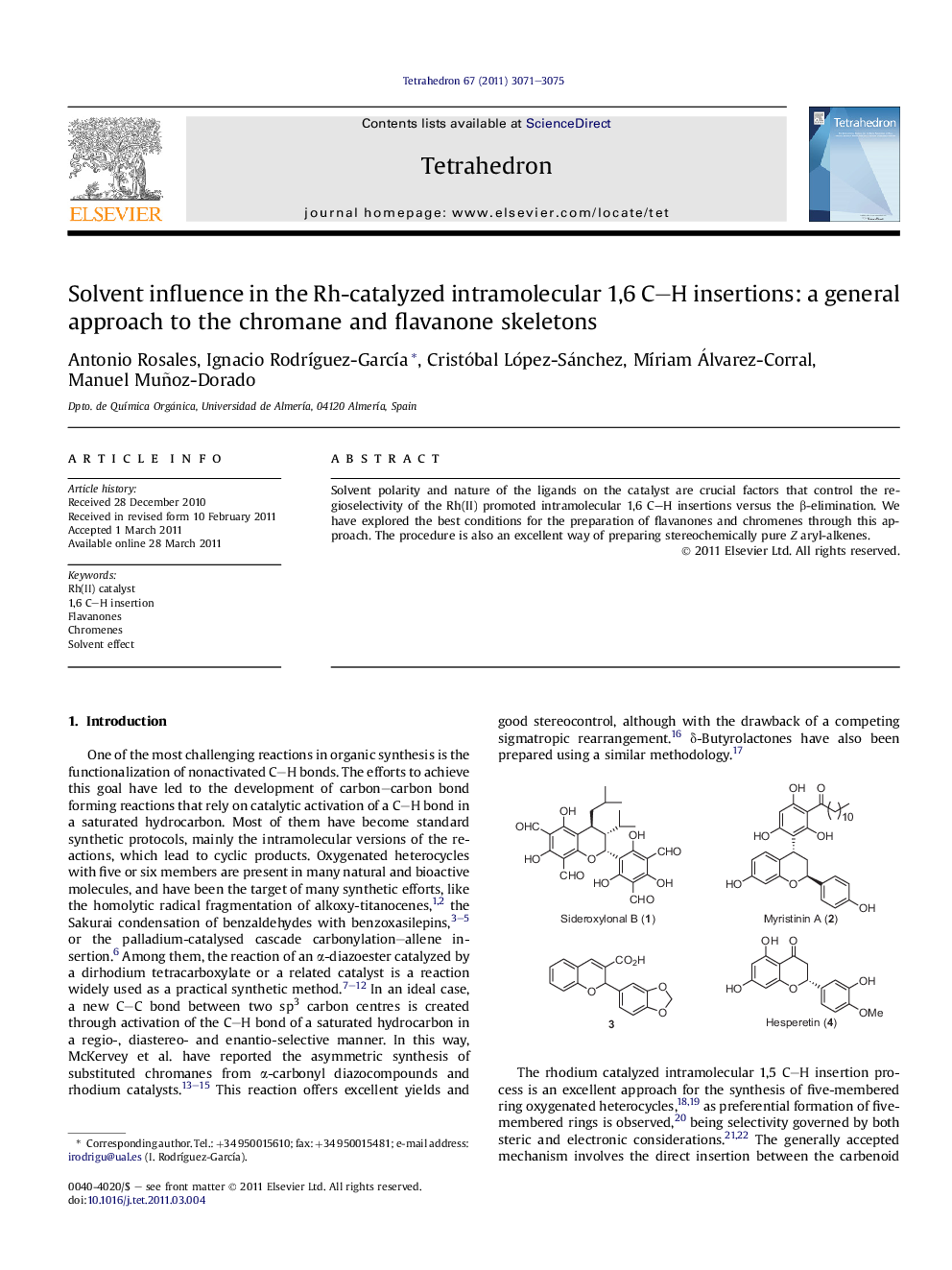
Solvent polarity and nature of the ligands on the catalyst are crucial factors that control the regioselectivity of the Rh(II) promoted intramolecular 1,6 C-H insertions versus the β-elimination. We have explored the best conditions for the preparation of flavanones and chromenes through this approach. The procedure is also an excellent way of preparing stereochemically pure Z aryl-alkenes.
Graphical abstractDownload full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Antonio Rosales, Ignacio RodrÃguez-GarcÃa, Cristóbal López-Sánchez, MÃriam Álvarez-Corral, Manuel Muñoz-Dorado,