| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5221471 | Tetrahedron | 2010 | 6 Pages |
Abstract
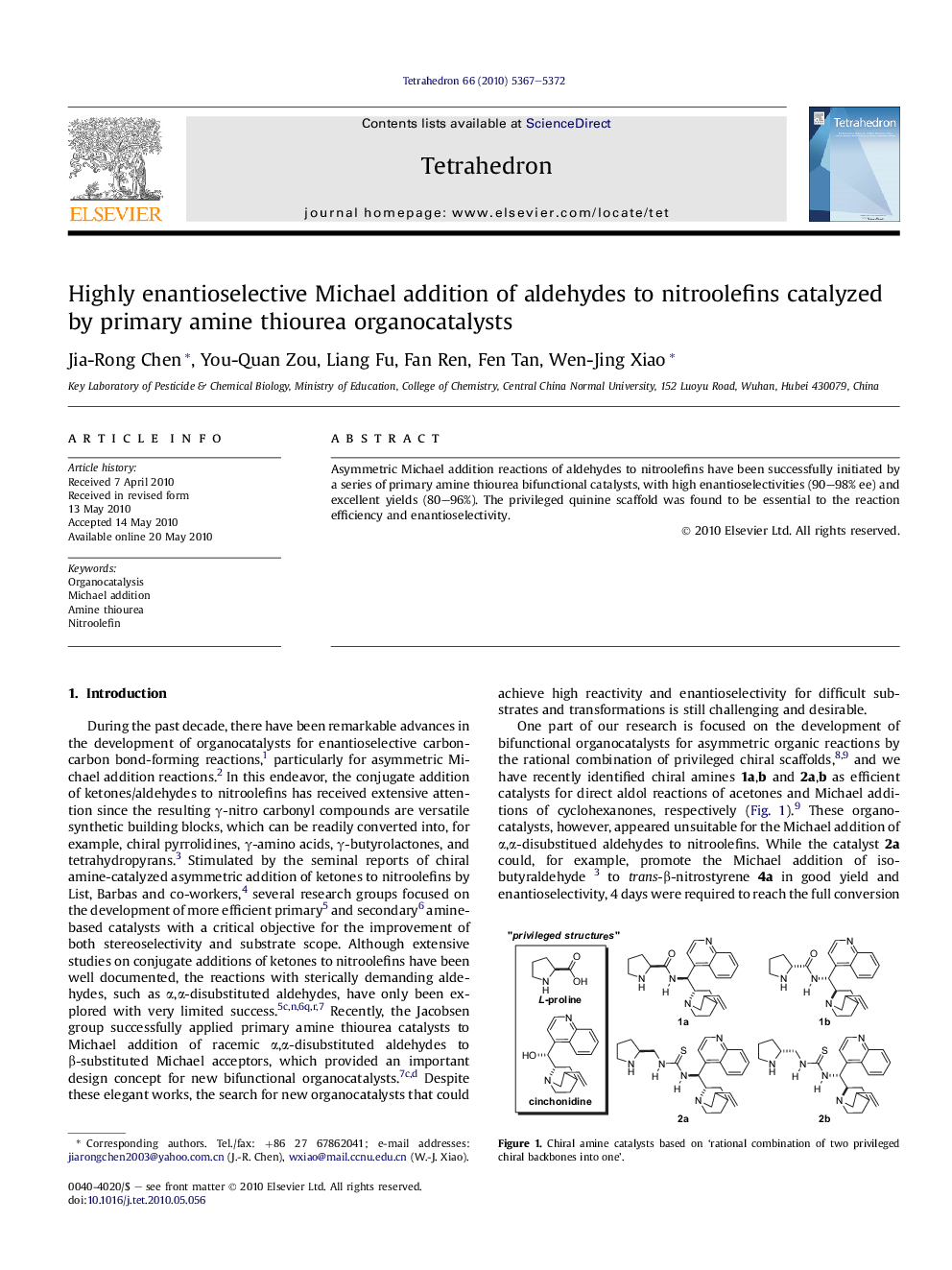
Asymmetric Michael addition reactions of aldehydes to nitroolefins have been successfully initiated by a series of primary amine thiourea bifunctional catalysts, with high enantioselectivities (90–98% ee) and excellent yields (80–96%). The privileged quinine scaffold was found to be essential to the reaction efficiency and enantioselectivity.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry