| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5222340 | Tetrahedron | 2009 | 7 Pages |
Abstract
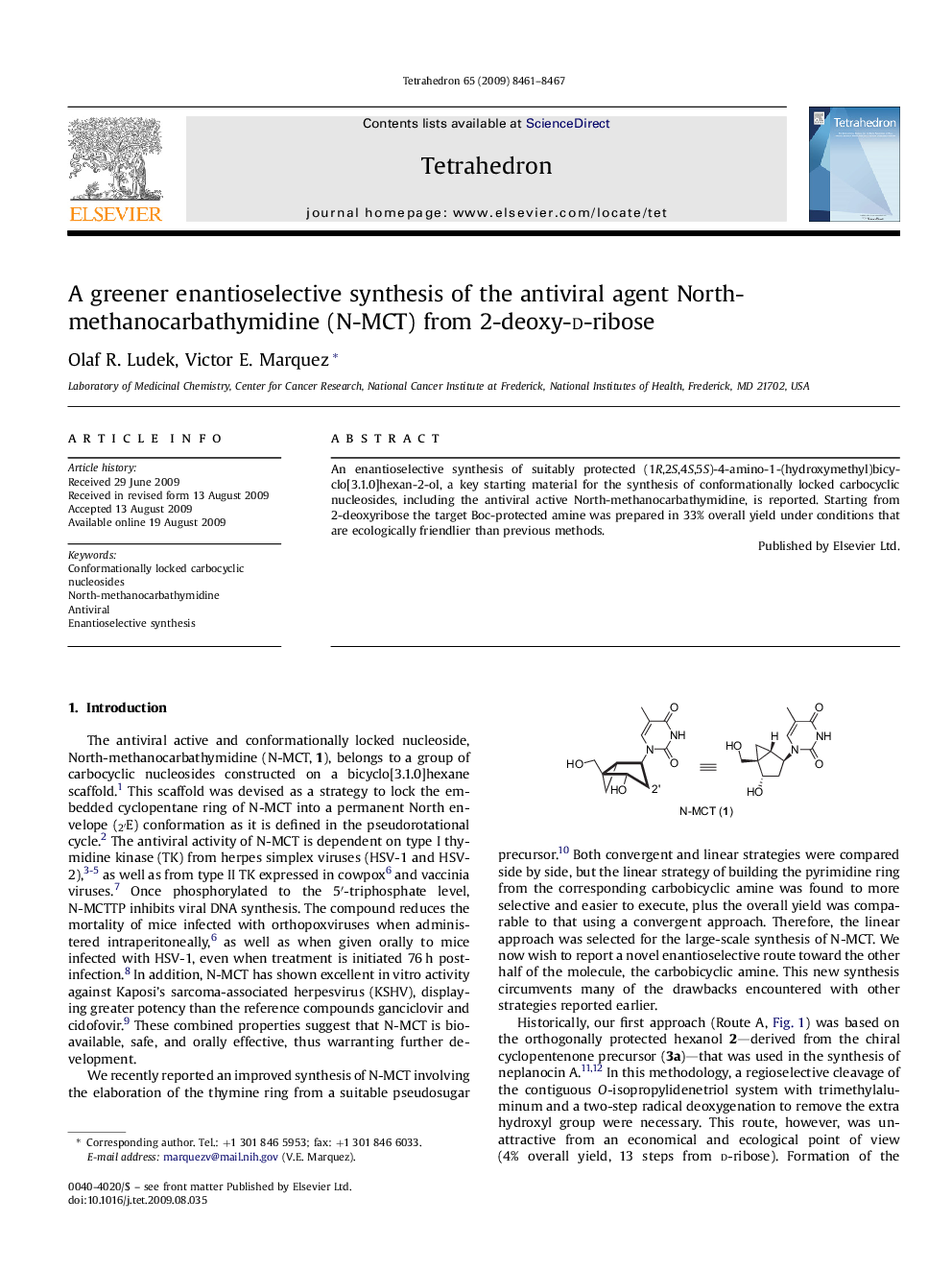
An enantioselective synthesis of suitably protected (1R,2S,4S,5S)-4-amino-1-(hydroxymethyl)bicyclo[3.1.0]hexan-2-ol, a key starting material for the synthesis of conformationally locked carbocyclic nucleosides, including the antiviral active North-methanocarbathymidine, is reported. Starting from 2-deoxyribose the target Boc-protected amine was prepared in 33% overall yield under conditions that are ecologically friendlier than previous methods.
Graphical abstractDownload full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Olaf R. Ludek, Victor E. Marquez,